4.2: Ways in which Chemical Control Agents Affect Bacteria
- Page ID
- 3155
Describe six different ways antibiotics or disinfectants may affect bacterial structures or macromolecules and state how each ultimately causes harm to the cell. State which of the following groups of antibiotics: 1) inhibit peptidoglycan synthesis; 2) inhibit nucleic acid synthesis; 3) alter bacterial 30S ribosomal subunits blocking translation; or 4) alter bacterial 50S ribosomal subunits blocking translation. - macrolides(erythromycin, azithromycin, clarithromycin, dirithromycin, troleandomycin, etc.), oxazolidinones (linezolid), and streptogramins
- penicillins, monobactams, carbapenems, cephalosporins, and vancomycin
- fluoroquinolones (norfloxacin, lomefloxacin, fleroxacin, ciprofloxacin, enoxacin, trovafloxacin, etc.), sulfonamides and trimethoprim, and metronidazole
- aminoglycosides (streptomycin, neomycin, netilmicin, tobramycin, gentamicin, amikacin, etc.) and tetracyclines (tetracycline, doxycycline, demeclocycline, minocycline, etc.)
State two modes of action for disinfectants, antiseptics, and sanitizers.
- macrolides(erythromycin, azithromycin, clarithromycin, dirithromycin, troleandomycin, etc.), oxazolidinones (linezolid), and streptogramins
- penicillins, monobactams, carbapenems, cephalosporins, and vancomycin
- fluoroquinolones (norfloxacin, lomefloxacin, fleroxacin, ciprofloxacin, enoxacin, trovafloxacin, etc.), sulfonamides and trimethoprim, and metronidazole
- aminoglycosides (streptomycin, neomycin, netilmicin, tobramycin, gentamicin, amikacin, etc.) and tetracyclines (tetracycline, doxycycline, demeclocycline, minocycline, etc.)
The basis of chemotherapeutic control of bacteria is selective toxicity. Selective toxicity means that the chemical being used should inhibit or kill the intended pathogen without seriously harming the host. A broad spectrum agent is one generally effective against a variety of Gram-positive and Gram-negative bacteria; a narrow spectrum agent generally works against just Gram-positives, Gram-negatives, or only a few bacteria. Such agents may be cidal or static in their action. A cidal agent kills the organism while a static agent inhibits the organism's growth long enough for body defenses to remove it. There are two categories of antimicrobial chemotherapeutic agents: antibiotics and synthetic drugs. Antibiotics are metabolic products of one microorganism that inhibit or kill other microorganisms. Synthetic drugs are antimicrobial drugs synthesized by chemical procedures in the laboratory. Many of today's antibiotics are now actually semisynthetic and some are even made synthetically. We will now look at the various ways in which our control agents affect bacteria altering their structures or interfering with their cellular functions.
Describe one way an antibiotic can inhibit peptidoglycan synthesis, state how that ultimately kills the bacterium, and give an example of such an antibiotic. Describe one way an antibiotic can alter bacterial ribosomes, state how that ultimately inhibits or kills the bacterium, and give an example of such an antibiotic. Describe one way an antibiotic can interfere with bacterial DNA synthesis, state how that ultimately kills the bacterium, and give an example of such an antibiotic.
Many Antibiotics inhibit Synthesis of Peptidoglycan and cause Osmotic Lysis
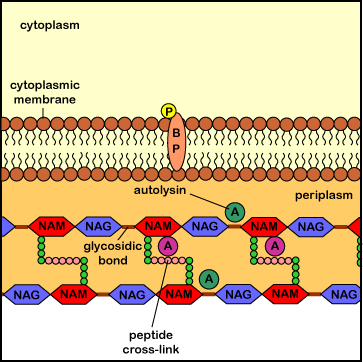
Interference with this process results in the formation of a weak cell wall and osmotic lysis of the bacterium. Agents that inhibit peptidoglycan synthesis include the penicillins (penicillin G, methicillin, oxacillin, ampicillin, amoxicillin, ticarcillin, etc.), the cephalosporins (cephalothin, cefazolin, cefoxitin, cefotaxime, cefaclor, cefoperazone, cefixime, ceftriaxone, cefuroxime, etc.), the carbapenems (imipenem, metropenem), the monobactems (aztreonem), and the carbacephems (loracarbef). Penicillins, monobactams, carbapenems, and cephalosporins are known chemically as beta-lactam antibiotics because they all share a molecular structure called a beta-lactam ring (see Figure \(\PageIndex{5}\)). The glycopeptides (vancomycin, teichoplanin) and lipopeptides (daptomycin) also inhibit peptidoglycan synthesis.
a. Beta lactam antibiotics such as penicillins and cephalosporins
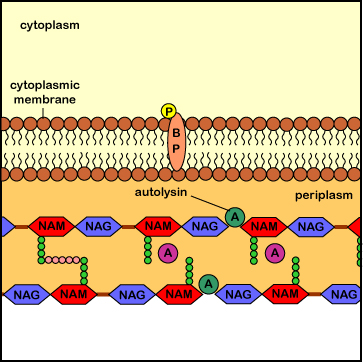
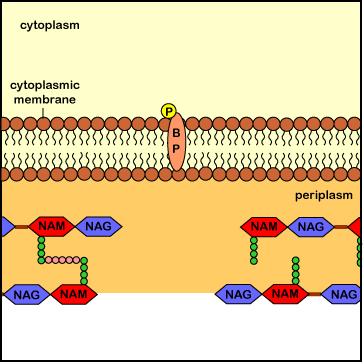
Penicillins, cephalosporins, as well as other beta-lactam antibiotics (see Common Antibiotics), bind to the transpeptidase enzymes (also called penicillin-binding proteins) responsible for reforming the peptide cross-links between rows and layers of peptidoglycan of the cell wall as new peptidoglycan monomers are added during bacterial cell growth. This binding blocks the transpeptidase enzymes from cross-linking the sugar chains and results in a weak cell wall. In addition, these antibiotics appear to interfere with the bacterial controls that keep autolysins in check, with resulting degradation of the peptidoglycan and osmotic lysis of the bacterium (see Figure \(\PageIndex{6}\)).
| Flash animation showing how penicillins inhibit peptidoglycan synthesis. © Juliet V. Spencer, Stephanie K.M. Wong, authors, Licensed for use, ASM MicrobeLibrary. |
| YouTube movie showing lysis of E. coli after exposure to a penicillin #1 |
| YouTube movie showing lysis of E. coli after exposure to a penicillin #2 |
b. Glycopeptides
Glycopeptides such as vancomycin (see Common Antibiotics) and the lipoglycopeptide teichoplanin bind to the D-Ala-D-Ala portion of the pentapeptides of the peptidoglycan monomers and block the formation of gycosidic bonds between the sugars by the transgycosidase enzymes, as well as the formation of the peptide cross-links by the transpeptidase enzymes. This results in a weak cell wall and subsequent osmotic lysis of the bacterium (see Figure \(\PageIndex{7}\)).
| Flash animation showing how vancomycin inhibit peptidoglycan synthesis. © Juliet V. Spencer, Stephanie K.M. Wong, authors, Licensed for use, ASM MicrobeLibrary. |
c. Bacitracin
Bacitracin (see Common Antibiotics) binds to the transport protein bactoprenol after it inserts a peptidoglycan monomer into the growing cell wall. It subsequently prevents the dephosphorylation of the bactoprenol after it releases the monomer it has transported across the membrane. Bactoprenol molecules that have not lost the second phosphate group cannot assemble new monomers and transport them across the cytoplasmic membrane. As a result, no new monomers are inserted into the growing cell wall. As the autolysins continue to break the peptide cross-links and new cross-links fail to form, the bacterium bursts from osmotic lysis (see Figure \(\PageIndex{8}\)).
| Flash animation showing how bacitracin inhibit peptidoglycan synthesis. © Juliet V. Spencer, Stephanie K.M. Wong, authors, Licensed for use, ASM MicrobeLibrary. |
A few antimicrobial chemotherapeutic agents inhibit normal synthesis of the acid-fast cell wall
A few antimicrobial chemotherapeutic agents inhibit normal synthesis of the acid-fast cell wall of the genus Mycobacterium (see Common Antibiotics).. INH(isoniazid) appears to block the synthesis of mycolic acid, a key component of the acid-fast cell wall of mycobacteria (see Figure \(\PageIndex{9}\)). Ethambutol interferes with the synthesis of the outer membrane of acid-fast cell walls (see Figure \(\PageIndex{9}\)).
A very few antibiotics alter the bacterial cytoplasmic membrane causing leakage of molecules and enzymes needed for normal bacterial metabolism.
A very few antibiotics, such as polymyxins, colistins, and daptomycin (Common Antibiotics), as well as many disinfectants and antiseptics, such as orthophenylphenol, chlorhexidine, hexachlorophene, zephiran, alcohol, and triclosans, alter the bacterial cytoplasmic membrane causing leakage of molecules and enzymes needed for normal bacterial metabolism.
- Polymyxins and colistins act as detergents and alter membrane permeability in Gram-negative bacteria. They cannot effectively diffuse through the thick peptidoglycan layer in gram-positives.
- Daptomycin disrupts the bacteria cytoplasmic membrane function by apparently binding to the membrane and causing rapid depolarization. This results on a loss of membrane potential and leads to inhibition of protein, DNA and RNA synthesis, resulting in bacterial cell death.
- Pyrazinamide inhibits fatty acid synthesis in the membranes of Mycobacterium tuberculosis.
Some antimicrobial chemotherapeutic agents inhibit normal nucleic acid replication in bacteria (see Common Antibiotics).
a. Fluoroquinolones
Fluoroquinolones (norfloxacin, lomefloxacin, fleroxacin, ciprofloxacin, enoxacin, trovafloxacin, gatifloxacin, etc., (Common Antibiotics))) work by inhibiting one or more of a group of enzymes called topoisomerase, enzymes needed for supercoiling, replication, and separation of circular bacterial DNA (see Figure \(\PageIndex{10}\)). For example, DNA gyrase (topoisomerase II) catalyzes the negative supercoiling of the circular DNA found in bacteria. It is critical in bacterial DNA replication, DNA repair, transcription of DNA into RNA, and genetic recombination. Topoisomerase IV, on the other hand, is involved in the relaxation of the supercoiled circular DNA, enabling the separation of the interlinked daughter chromosomes at the end of bacterial DNA replication.
In Gram-negative bacteria, the main target for fluoroquinolones is DNA gyrase (topoisomerase II), an enzyme responsible for supercoiling of bacterial DNA during DNA replication; in Gram-positive bacteria, the primary target is topoisomerase IV, an enzyme responsible for relaxation of supercoiled circular DNA and separation of the inter-linked daughter chromosomes.
b. Sulfonamides
Sulfonamides (sulfamethoxazole, sulfanilamide) and diaminopyrimidines (trimethoprim) (see Common Antibiotics) block enzymes in the bacteria pathway required for the synthesis of tetrahydrofolic acid, a cofactor needed for bacteria to make the nucleotide bases thymine, guanine, uracil, and adenine (see Figure \(\PageIndex{11}\)).
This is done through a process called competitive antagonism whereby a drug chemically resembles a substrate in a metabolic pathway. Because of their similarity, either the drug or the substrate can bind to the substrate's enzyme. While the enzyme is bound to the drug, it is unable to bind to its natural substrate and that blocks that step in the metabolic pathway (see Figure \(\PageIndex{12}\)). Typically, a sulfonamide and a diaminopyrimidine are combined. Co-trimoxazole, for example, is a combination of sulfamethoxazole and trimethoprim.
Sulfonamides such as sulfamethoxazole tie up the first enzyme in the pathway, the conversion of para-aminobenzoic acid to dihydropteroic acid (see Figure \(\PageIndex{11}\)). Trimethoprim binds to the third enzyme in the pathway, an enzyme that is responsible for converting dihydrofolic acid to tetrahydrofolic acid (see Figure \(\PageIndex{11}\)). Without the tetrahydrofolic acid, the bacteria cannot synthesize DNA or RNA.
c. Metronidazole
Metronidazole (see Common Antibiotics) is a drug that is activated by the microbial proteins flavodoxin and feredoxin found in microaerophilc and anaerobic bacteria and certain protozoans. Once activated, the metronidazole puts nicks in the microbial DNA strands.
d. Rifampin
Rifampin (rifamycin) (see Common Antibiotics) blocks transcription by inhibiting bacterial RNA polymerase, the enzyme responsible for transcription of DNA to mRNA.
Many antibiotics alter bacterial ribosomes, interfering with translation of mRNA into proteins and thereby causing faulty protein synthesis (see Common Antibiotics).
To learn more detail about the specific steps involved in translation during bacterial protein synthesis, see the animation that follows. Protein synthesis is discussed in greater detail in Unit 6.
a. Aminoglycosides
The aminoglycosides (streptomycin, neomycin, netilmicin, tobramycin, gentamicin, amikacin, etc. (see Common Antibiotics)) bind irreversibly to the 16S rRNA in the 30S subunit of bacterial ribosomes. Although the exact mechanism of action is still uncertain, there is evidence that some prevent the transfer of the peptidyl tRNA from the A-site to the P-site, thus preventing the elongation of the polypeptide chain. Some aminoglycosides also appear to interfere with the proofreading process that helps assure the accuracy of translation (see Figure \(\PageIndex{13}\)). Possibly the antibiotics reduce the rejection rate for tRNAs that are near matches for the codon. This leads to misreading of the codons or premature termination of protein synthesis (see Figure \(\PageIndex{14}\)). Aminoglycosides may also interfere directly or indirectly with the function of the bacterial cytoplasmic membrane. Because of their toxicity, aminoglycosides are generally used only when other first line antibiotics are not effective.
b. Tetracyclines
The tetracyclines (tetracycline, doxycycline, demeclocycline, minocycline, etc. (see Common Antibiotics)) bind reversibly to the 16S rRNA in the 30S ribosomal subunit, distorting it in such a way that the anticodons of charged tRNAs cannot align properly with the codons of the mRNA (see Figure \(\PageIndex{15}\)).
c. Macrolides
The macrolides (erythromycin, azithromycin, clarithromycin, dirithromycin, troleandomycin, etc. (see Common Antibiotics)) bind reversibly to the 23S rRNA in the 50S subunit of bacterial ribosomes. They appear to inhibit elongation of the protein by preventing the enzyme peptidyltransferase from forming peptide bonds between the amino acids (see Figure \(\PageIndex{16}\)). They may also prevent the transfer of the peptidyl tRNA from the A-site to the P-site (see Figure \(\PageIndex{17}\)) as the beginning peptide chain on the peptidyl tRNA adheres to the ribosome, creates friction, and blocks the exit tunnel of the 50S ribosomal subunit.
d. Oxazolidinones
The oxazolidinones (linezolid, sivextro) (see Common Antibiotics), following the first cycle of protein synthesis, interfere with translation sometime before the initiation phases. They appear to bind to the 50S ribosomal subunit and interfere with its binding to the initiation complex (see Figure \(\PageIndex{18}\)).
e. Streptogramins
The streptogramins (synercid, a combination of quinupristin and dalfopristin (see Common Antibiotics)) bind to two different locations on the 23S rRNA in the 50S ribosomal subunit and work synergistically to block translation. There are reports that the streptogramins may inhibit the attachment of the charged tRNA to the A-site or may block the peptide exit tunnel of the 50S ribosomal subunit.
For a more detailed description of any specific antimicrobial agent, see the website of RxList - The Internet Drug Index.
Modes of action for disinfectants, antiseptics, and sanitizers
Disinfection is the elimination of microorganisms, but not necessarily endospores, from inanimate objects or surfaces, whereas decontamination is the treatment of an object or inanimate surface to make it safe to handle. Sterilization is the process of destroying all living organisms and viruses. A sterile object is one free of all life forms, including bacterial endospores, as well as viruses.
The term disinfectant is used for an agent used to disinfect inanimate objects or surfaces but is generally too toxic to use on human tissues. An antiseptic refers to an agent that kills or inhibits growth of microbes but is safe to use on human tissue. A sanitizer describes an agent that reduces microbial numbers to a safe level. Because disinfectants and antiseptics often work slowly on some viruses - such as the hepatitis viruses, bacteria with an acid-fast cell wall such as Mycobacterium tuberculosis, and especially bacterial endospores, produced by the genus Bacillus and the genus Clostridium, they are usually unreliable for sterilization - the destruction of all life forms.
There are a number of factors which influence the antimicrobial action of disinfectants and antiseptics, including:
- The concentration of the chemical agent.
- The temperature at which the agent is being used. Generally, the lower the temperature, the longer it takes to disinfect or decontaminate.
- The kinds of microorganisms present. Endospore producers such as Bacillus species, Clostridium species, and acid-fast bacteria like Mycobacterium tuberculosis are harder to eliminate.
- The number of microorganisms present. The more microorganisms present, the harder it is to disinfect or decontaminate.
- The nature of the material bearing the microorganisms. Organic material such as dirt and excreta interferes with some agents.
The best results are generally obtained when the initial microbial numbers are low and when the surface to be disinfected is clean and free of possible interfering substances.
There are 2 common antimicrobial modes of action for disinfectants, antiseptics, and sanitizers:
1. They may damage the lipids and/or proteins of the semipermeable cytoplasmic membrane of microorganisms resulting in leakage of cellular materials needed to sustain life.
2. They may denature microbial enzymes and other proteins, usually by disrupting the hydrogen and disulfide bonds that give the protein its three-dimensional functional shape. This blocks metabolism.
A large number of such chemical agents are in common use. Some of the more common groups are listed below:
1. Phenol and phenol derivatives: Phenol (5-10%) was the first disinfectant commonly used. However, because of its toxicity and odor, phenol derivatives (phenolics) are now generally used. The most common phenolic is orthophenylphenol, the agent found in O-syl®, Staphene®, and Amphyl®. Bisphenols contain two phenolic groups and typically have chlorine as a part of their structure. They include hexachlorophene and triclosan. Hexachlorophene in a 3% solution is combined with detergent and is found in PhisoHex®. Triclosan is an antiseptic very common in antimicrobial soaps and other products. Biguanides include chlorhexadine and alexidine. A 4% solution of chlorhexidine in isopropyl alcohol and combined with detergent (Hibiclens® and Hibitane®) is a common hand washing agent and surgical handscrub. These agents kill most bacteria, most fungi, and some viruses, but are usually ineffective against endospores. Chloroxylenol (4-chloro-3,5-dimethylphenol) is a broad spectrum antimicrobial chemical compound used to control bacteria, algae, fungi and virus and is often used in antimicrobial soaps and antiseptics. Phenol and phenolics alter membrane permeability and denature proteins. Bisphenols, biguanides, and chloroxylenol alter membrane permeability.
2. Soaps and detergents: Soaps are only mildly microbicidal. Their use aids in the mechanical removal of microorganisms by breaking up the oily film on the skin (emulsification) and reducing the surface tension of water so it spreads and penetrates more readily. Some cosmetic soaps contain added antiseptics to increase antimicrobial activity.
Detergents may be anionic or cationic. Anionic (negatively charged) detergents, such as laundry powders, mechanically remove microorganisms and other materials but are not very microbicidal. Cationic (positively charged) detergents alter membrane permeability and denature proteins. They are effective against many vegetative bacteria, some fungi, and some viruses. However, bacterial endospores and certain bacteria such as Mycobacterium tuberculosis and Pseudomonas species are usually resistant. Soaps and organic materials like excreta also inactivate them. Cationic detergents include the quaternary ammonium compounds such as benzalkonium chloride, zephiran®, diaprene, roccal, ceepryn, and phemerol. Household Lysol® contains alkyl dimethyl benzyl ammonium chloride and alcohols.
3. Alcohols
70% solutions of ethyl or isopropyl alcohol are effective in killing vegetative bacteria, enveloped viruses, and fungi. However, they are usually ineffective against endospores and non-enveloped viruses. Once they evaporate, their cidal activity will cease. Alcohols denature membranes and proteins and are often combined with other disinfectants, such as iodine, mercurials, and cationic detergents for increased effectiveness.
4. Acids and alkalies
Acids and alkalies alter membrane permeability and denature proteins and other molecules. Salts of organic acids, such as calcium propionate, potassium sorbate, and methylparaben, are commonly used as food preservatives. Undecylenic acid (Desenex®) is used for dermatophyte infections of the skin. An example of an alkali is lye (sodium hydroxide).
5. Heavy metals
Heavy metals, such as mercury, silver, and copper, denature proteins. Mercury compounds (mercurochrome, metaphen, merthiolate) are only bacteriostatic and are not effective against endospores. Silver nitrate (1%) is sometimes put in the eyes of newborns to prevent gonococcal ophthalmia. Copper sulfate is used to combat fungal diseases of plants and is also a common algicide. Selinium sulfide kills fungi and their spores.
6. Chlorine
Chlorine gas reacts with water to form hypochlorite ions, which in turn denature microbial enzymes. Chlorine is used in the chlorination of drinking water, swimming pools, and sewage. Sodium hypochlorite is the active agent in household bleach. Calcium hypochlorite, sodium hypochlorite, and chloramines (chlorine plus ammonia) are used to sanitize glassware, eating utensils, dairy and food processing equipment, hemodialysis systems, and treating water supplies.
7. Iodine and iodophores
Iodine also denatures microbial proteins. Iodine tincture contains a 2% solution of iodine and sodium iodide in 70% alcohol. Aqueous iodine solutions containing 2% iodine and 2.4% sodium iodide are commonly used as a topical antiseptic. Iodophores are a combination of iodine and an inert polymer such as polyvinylpyrrolidone that reduces surface tension and slowly releases the iodine. Iodophores are less irritating than iodine and do not stain. They are generally effective against vegetative bacteria, Mycobacterium tuberculosis, fungi, some viruses, and some endospores. Examples include Wescodyne®, Ioprep®, Ioclide®, Betadine®, and Isodine®.
8. Aldehydes
Aldehydes, such as formaldehyde and glutaraldehyde, denature microbial proteins. Formalin (37% aqueous solution of formaldehyde gas) is extremely active and kills most forms of microbial life. It is used in embalming, preserving biological specimens, and in preparing vaccines. Alkaline glutaraldehyde (Cidex®), acid glutaraldehyde (Sonacide®), and glutaraldehyde phenate solutions (Sporocidin®) kill vegetative bacteria in 10-30 minutes and endospores in about 4 hours. A 10 hour exposure to a 2% glutaraldehyde solution can be used for cold sterilization of materials. Ortho-phthalaldehyde (OPA) is dialdehyde used as a high-level disinfectant for medical instruments.
9. Peroxygens
Peroxygens are oxidizing agents that include hydrogen peroxide and peracetic acid. Hydrogen peroxide is broken down into water and oxygen by the enzyme catalase in human cells and is not that good of an antiseptic for open wounds but is useful for disinfecting inanimate objects. The high concentrations of hydrogen peroxide overwhelm the catalase found in microbes. Peracetic acid is a disinfectant that kills microorganisms by oxidation and subsequent disruption of their cytoplasmic membrane. It is widely used in health care, food processing, and water treatment.
10. Ethylene oxide gas
Ethylene oxide is one of the very few chemicals that can be relied upon for sterilization (after 4-12 hours exposure). Since it is explosive, it is usually mixed with inert gases such as freon or carbon dioxide. Gaseous chemosterilizers, using ethylene oxide, are commonly used to sterilize heat-sensitive items such as plastic syringes, petri plates, textiles, sutures, artificial heart valves, heart-lung machines, and mattresses. Ethylene oxide has very high penetrating power and denatures microbial proteins. Vapors are toxic to the skin, eyes, and mucous membranes and are also carcinogenic. Another gas that is used as a sterilant is chlorine dioxide which denatures proteins in vegetative bacteria, bacterial endospores, viruses, and fungi.
Summary
- Many antibiotics (penicillins, cephalosporins, vancomycin, bacitracin) inhibit normal synthesis of peptidoglycan by bacteria and cause osmotic lysis. They do this by inactivating the enzymes or the transporters involved in peptidoglycan synthesis.
- A few antimicrobial chemotherapeutic agents (INH, ethambutol) inhibit normal synthesis of the acid-fast cell wall.
- A very few antibiotics (polymyxin, colistin, daptomycin) alter the bacterial cytoplasmic membrane causing leakage of molecules and enzymes needed for normal bacterial metabolism.
- Some antimicrobial chemotherapeutic agents (fluoroquinolones, sulfonamides, trimethoprim) inhibit normal nucleic acid replication in bacteria.
- Many antibiotics (tetracyclines, macrolides, oxazolidinones, streptogramins) alter bacterial ribosomes, interfering with translation of mRNA into proteins and thereby causing faulty protein synthesis.
- There are 2 common antimicrobial modes of action for disinfectants, antiseptics, and sanitizers: damaging the lipids and/or proteins of the semipermeable cytoplasmic membrane of microorganisms resulting in leakage of cellular materials; and denaturing microbial enzymes and other proteins.
- A number of factors which influence the antimicrobial action of disinfectants and antiseptics, including the concentration of the chemical agent, the temperature at which the agent is being used, the kinds of microorganisms present, the number of microorganisms present, and the nature of the material bearing the microorganisms.
- Endospore producers such as Bacillus species, Clostridium species, and acid-fast bacteria like Mycobacterium tuberculosis are harder to eliminate.


