12.3G: Natural Killer Cells (NK Cells)
- Page ID
- 3300
Briefly describe how NK cells bind to and kill infected cells and tumor cells through ADCC. Briefly describe how NK cells recognize and kill infected cells and tumor cells that suppress MHC-I production.
NK cells are another group of cytolytic lymphocytes that are distinct from B-lymphocytes and T-lymphocytes, and participate in both innate immunity and adaptive immunity. NK cells are lymphocytes that lack B-cell receptors and T-cell receptors. They are designed to kill certain mutant cells and virus-infected cells in one of two ways:
Antibody-dependent Cellular Cytotoxicity
NK cells kill cells to which antibody molecules have attached through a process called antibody-dependent cellular cytotoxicity (ADCC) as shown in Figure \(\PageIndex{1}\), Figure \(\PageIndex{2}\), and Figure \(\PageIndex{3}\). The Fab portion of the antibody binds to epitopes on the "foreign" cell. The NK cell then binds to the Fc portion of the antibody. The NK cell is then able to contact the cell and by inducing a programmed cell suicide called apoptosis.
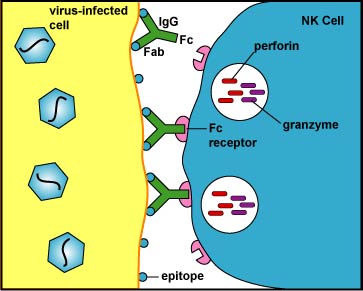
As a result, the infected cell breaks into membrane-bound fragments that are subsequently removed by phagocytes. If very large numbers of perforins are inserted into the plasma membrane of the infected cell, this can result in a weakening of the membrane and lead to cell lysis rather than apoptosis. An advantage to killing infected cells by apoptosis is that the cell's contents, including viable virus particles and mediators of inflammation, are not released as they are during cell lysis.
Innate Immunity
In addition, NK cells produce a variety of cytokines, including proinflammatory cytokines, chemokines, colony-stimulating factors, and other cytokines that function as regulators of body defenses. For example, through cytokine production NK cells also suppress and/or activate macrophages, suppress and/or activate the antigen-presenting capabilities of dendritic cells, and suppress and/or activate T-lymphocyte responses.
Summary
- Natural Killer (NK) cells are able to recognize infected cells, cancer cells, and stressed cells and kill them. In addition, they produce a variety of cytokines, including proinflammatory cytokines, chemokines, colony-stimulating factors, and other cytokines that function as regulators of body defenses.
- NK cells play a role in adaptive immune responses by way of antibody-dependent cellular cytotoxicity or ADCC where they bind to and kill cells to which antibody molecules have bound.
- During ADCC, the Fab portion of the antibody binds to epitopes on the "foreign" cell. The NK cell then binds to the Fc portion of the antibody and the NK cell is then able to contact and kill the cell by inducing a programmed cell suicide called apoptosis.
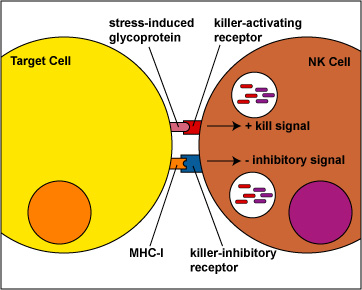
- During innate immunity, NK cells use a dual receptor system in determining whether to kill or not kill human cells.
- When body cells are either under stress, are turning into tumors, or are infected, various stress-induced molecules are produced and are put on the surface of that cell.
- The first receptor, called the killer-activating receptor, can bind to these stress-induced molecules, and this sends a positive signal that enables the NK cell to kill the cell to which it has bound unless the second receptor cancels that signal.
- The second receptor, called the killer-ihibitory receptor, recognizes MHC-I molecules that are usually present on all nucleated human cells. If MHC-I molecules/self peptide complexes are expressed on the cell, the killer-inhibitory receptors on the NK cell recognize this MHC-I/peptide complex and sends a negative signal that overrides the original kill signal and prevents the NK cell from killing the cell to which it has bound.
- NK cells kill their target cells by inducing apoptosis, a programmed cell suicide.


