12.3C: T4-Lymphocytes (T4-Cells)
- Page ID
- 3296
Describe the overall function of T4-lymphocytes and their activation in terms of the following: - the role of their TCRs and CD4 molecules
- what they recognize on antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B-lymphocytes.
- the role of antigen-presenting dendritic cells in the activation of naive T4-lymphocytes.
Compare TH1, TH2, TH17, Treg, and TFH lymphocytes in terms of their primary function(s) in immunity.
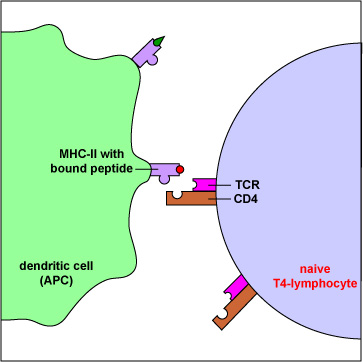
- To view an electron micrograph of a dendritic cell presenting antigen to T-lymphocytes, #1 see the Web page for the University of Illinois College of Medicine.
- To view an electron micrograph of a dendritic cell presenting antigen to T-lymphocytes, #2 see the Web page for the University of Illinois College of Medicine.
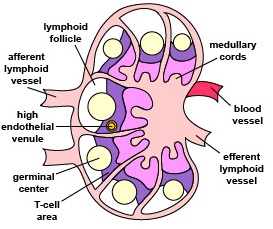
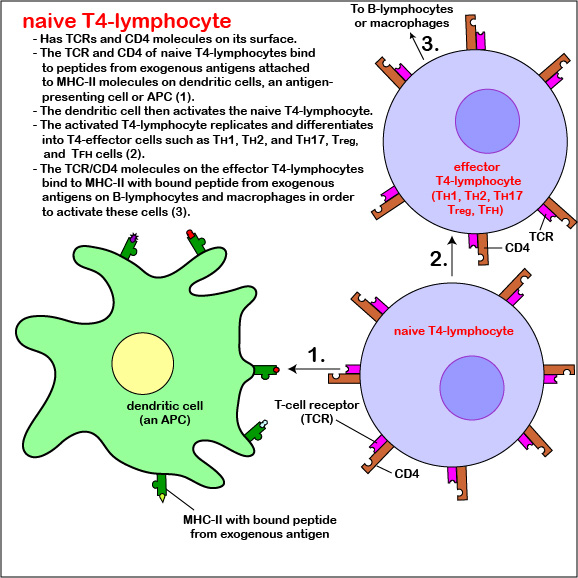
Those naive T4-lymphocytes not activated by epitopes of antigens on the dendritic cells exit the lymph node (or other lymphoid tissue) and eventually re-enter the bloodstream. However, if a TCR and CD4 molecule of the naive T4-lymphocyte detects a corresponding MHC-II/peptide complex on a mature dendritic cell, this will send a first signal for the activation of that naive T-lymphocyte. Next, a second signal that promotes survival of that T-lymphocyte is sent when co-stimulatory molecules such as B7.1 and B7.2 on the dendritic cell bind to CD28 molecules on the T4-lymphocyte. Finally, the dendritic cell produces cytokines such as interleukin-6 (IL-6), IL-4, IL-12, and T-cell growth factor-beta (TGF-ß) that contribute to proliferation of the T4-lymphocytes and their differentiation into effector T4-lymphocytes, the cells the body uses to regulate both humoral immunity and cell-mediated immunity through the cytokines they produce. (Activated T4-lymphocytes remain in the lymph node as they proliferate (clonal expansion) and only leave the lymphoid tissues and re-enter the bloodstream after they have differentiated into effector T4-lymphocytes.)
CD28-dependent co-stimulation of the T4-lymphocyte also stimulates it to synthesize the cytokine interleukin-2 (IL-2) as well as a high-affinity IL-2 receptor. The binding of IL-2 to its high affinity receptor allows for cell proliferation and formation of a clone of thousands of identical T4-lymphocytes after several days. IL-2 also contributes to survival of those activated T4-lymphocytes and their differentiation into T4-effector cells. In addition, some of the T4-lymphocytes differentiate into circulating T4-memory cells. Circulating T4-memory cells allow for a more rapid and greater production of effector T4-lymphocytes upon subsequent exposure to the same antigen.
Differentiation of naive T4-lymphocyte into T4-effector lymphocytes
Functionally, there are many different types or subpopulations of effector T4-lymphocytes based on the cytokines they produce. Immune reactions are typically dominated by five primary types: TH1 cells, TH2 cells, TH17 cells, Treg cells, and TFH cells.
CD4 TH1 cells
Coordinate immunity against intracellular bacteria and promote opsonization. They:
- Produce cytokines such as interferon-gamma (IFN-?) that promote cell-mediated immunity against intracellular pathogens, especially by activating macrophages that have either ingested pathogens or have become infected with intracellular microbes such as Mycobacterium tuberculosis, Mycobacterium leprae, Leishmania donovani, and Pneumocystis jjroveci that are able to grow in the endocytic vesicles of macrophages. Activation of the macrophage by TH1 cells greatly enhances their antimicrobial effectiveness.
- They produce cytokines that promote the production of opsonizing antibodies that enhance phagocytosis (Figure \(\PageIndex{9}\)).
- Produce receptors that bind to and kill chronically infected cells, releasing the bacteria that were growing within the cell so the can be engulfed and killed by macrophages.
- Produce the cytokine interleukin-2 (IL-2) that induces T-lymphocyte proliferation.
- Produce cytokines such as tumor necrosis factor-alpha (TNF-a) that promote diapedesis of macrophages.
- Produces the chemokine CXCL2 to attract macrophages to the infection site.
- Produce cytokines that block the production of TH2 cells.
CD4 TH2 cells
Coordinate immunity against helminths and microbes that colonize mucous membranes
- Produce the cytokine interleukin-4 (IL-4) that promotes the production of the antibody isotype IgE in response to helminths and allergens. IgE is able to stick eosinophils to helminths for extracellular killing of the helminth (Figure \(\PageIndex{10}\)); it also promotes many allergic reactions.
- Produce cytokines that attract and activate eosinophils and mast cells.
- Promote the production of antibodies that neutralize microbes (Figure \(\PageIndex{11}\)) and toxins (Figure \(\PageIndex{12}\)) preventing their attachment to host cells.
- Produce cytokines that function as B-lymphocyte growth factors such as IL-4, IL-5, IL-9. and IL-13 (Figure \(\PageIndex{13}\)).
- Produce interleukin-22 (IL-22) that promotes the removal of microbes in mucosal tissues.
- Produce cytokines that block the production of TH1 cells.
CD4 TH17 cells
Promote a local inflammatory response to stimulate a strong neutrophil response and promote the integrity of the skin and mucous membranes
Produce cytokines like interleukin-17 (IL-17) and interleukin-6 (IL-6) that trigger local epithelial cells and fibroblasts to produce chemokines that recruit neutrophils to remove extracellular pathogens.
CD4 Treg cells
Suppress immune responses
- Produce inhibitory cytokines such as Interleukin-10 (IL-10) and TGF-ß that help to limit immune responses and prevent autoimmunity by suppressing T-lymphocyte activity.
- Promoting anamnestic response (immunologic memory) to resist repeat infections by the same microbe.
- Protecting beneficial normal flora in the intestines from being destroyed by the immune system.
- Aiding in sustaining pregnancy so that the immune system doesn't recognize a fetus as foreign and try to destroy it.
- Controlling established inflammation in tissues.
TFH cells
Promote humoral immunity by stimulating antibody production and antibody isotype switching by B-lymphocytes
- T follicular helper cells (TFH cells) are located in lymphoid follicles.
- TFH cells are now thought to be the primary effector T-lymphocytes that stimulate antibody production and isotype switching by B-lymphocytes. They are able to produce cytokines that are characteristic of both TH2 cells and TH1 cells.
- TFH cells producing (IFN-?) promote the production of opsonizing antibodies; those producing IL-4 promote the production of IgE.
Regulation of effector T4-lymphocyte activity: A role for commensals and helminths?
It is now recognized that genes associated with the normal flora ( microbiota) of the intestinal tract aid in digestion of many foods (especially plant polysaccharides that would normally be indigestible by humans), may play a role in normal growth and regulating appetite, and also help to regulate immune defenses. There is ever growing evidence that commensal bacteria of the gastrointestinal tract, as well as parasitic gastrointestinal helminths, may have coevolved with the human body over the past 200,000 year in such a way that genes from the human microbiota may play a significant role in regulating the human immune responses by providing a series of checks and balances that prevent the immune system from being too aggressive and causing an autoimmune attack upon the body's own cells, while still remaining aggressive enough to recognize and remove harmful pathogens. As exposure to and colonization with these once common human organisms has drastically changed over time as a result of less exposure to mud, animal and human feces,and helminth ova, coupled with ever increasing antibiotic use, improved sanitation, changes in the human diet, increased rate of cesarean sections, and improved methods of processing and preserving of food, the rate of allergies, allergic asthma, and autoimmune diseases (inflammatory bowel disease, Crone's disease, type-1 diabetes, and multiple sclerosis for example) has dramatically increased in developed countries while remaining relatively low in undeveloped and more agrarian parts of the world.
Numerous experiments in germ-free mice (mice with no intestinal commensals) have shown them to be much more susceptible to allergic asthma and autoimmune diseases such as colitis then normal mice. Feeding commensals or nematode ova to newborn germ-free mice, in turn, reduces the occurrence to these disorders. An imbalance in the relationship between proinflammatory TH17 cells and inflammation-suppressing Treg cells appears to increase the risk of inflammatory autoimmune diseases, while an imbalance between TH1 and TH2 cells seems to contribute to the risk of allergies and asthma. For example, a common commensal colon bacterium Bacteroides fragilis produces a molecule called polysaccharide A that dendritic cells engulf, process and present to naive T4-lymphocytes. This interaction stimulates the differentiation of the naive T4-lymphocytes into anti-inflammatory Treg cells that suppress the activity of proinflammatory TH17 cells. Without colonization with B. fragilis, the proinflammatory TH17 cells are not suppressed and there is an increased risk of inflammatory autoimmune diseases.
Normal intestinal microbiota also appear to regulate the intestinal levels of the invariant natural killer (iNKT) cells discussed under innate immune responses in Unit 4. iNKT cells recognize endogenous and exogenous lipid antigens presented on CD1d molecules by dendritic cells and in response, secrete proinflammatory cytokines. Germ free mice show an accumulation of iNKT cells in the colon and in the lungs and have an increased risk of intestinal bowel disease and allergic asthma. Neonatal germ free mice that were subsequently colonized with normal microbiota were protected from this iNKT cell accumulation and the resulting inflammatory pathology.
Summary
- T-lymphocytes refer to lymphocytes that are produced in the bone marrow but require interaction with the thymus for their maturation.
- The primary role of T4-lymphocytes is to regulate the body's immune responses through the production of cytokines.
- T4-lymphocytes display CD4 molecules and T-cell receptors (TCRs) on their surface.
- The TCR on T4-lymphocytes, in cooperation with CD4, typically bind peptides from exogenous antigens bound to MHC-II molecules.
- During its development, each T4-lymphocyte becomes genetically programmed to produce a TCR with a unique specificity that is able to bind an epitope/MHC-II complex on an APC such as a dendritic cell, a macrophage, or a B-lymphocyte possessing a corresponding shape.
- To become activated, naive T4-lymphocytes migrate through lymph nodes where the TCRs on the T4-lymphocyte are able to sample large numbers of MHC-II/peptide complexes on the antigen-presenting dendritic cells for ones that “fit”, thus enabling activation of that naïve T4-lymphocyte.
- After activation, the dendritic cell produces cytokines that contribute to proliferation of the T4-lymphocytes and their differentiation into effector T4-lymphocytes, the cells the body uses to regulate both humoral immunity and cell-mediated immunity through the cytokines they produce.
- Some of the T4-lymphocytes differentiate into circulating T4-memory cells that enable a more 9.rapid and greater production of effector T4-lymphocytes upon subsequent exposure to the same antigen.
- Functionally, there are many different types or subpopulations of effector T4-lymphocytes based on the cytokines they produce. Immune reactions are typically dominated by five primary types: TH1 cells, TH2 cells, TH17 cells, Treg cells, and TFH cells.
- CD4 TH1 cells coordinate immunity against intracellular bacteria and promote opsonization.
- CD4 TH2 cells coordinate immunity against helminths and microbes that colonize mucous membranes.
- CD4 TH17 cells promote a local inflammatory response to stimulate a strong neutrophil response and promote the integrity of the skin and mucous membranes.
- CD4 Treg cells suppress immune responses.
- TFH cells promote humoral immunity by stimulating antibody production and antibody isotype switching by B-lymphocytes.
- There is ever growing evidence that commensal bacteria of the gastrointestinal tract, as well as parasitic gastrointestinal helminths, may have coevolved with the human body over the past 200,000 year in such a way that genes from the human microbiota may play a significant role in regulating the human immune responses by providing a series of checks and balances that prevent the immune system from being too aggressive and causing an autoimmune attack upon the body's own cells, while still remaining aggressive enough to recognize and remove harmful pathogens.


