B2. Multi-Step Reactions
- Page ID
- 5071
Reversible First Order Reactions
\[ A \underset{k_2} {\overset{k_1}{\rightleftharpoons}} P \]
A differential equation can be written for this reaction:
\[ v = \dfrac{d[A]}{dt} = -k_1[A] + k_2[P] \label{7}\]
This can be solved through integration to give the following equations:
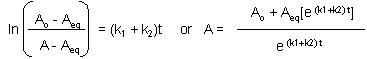
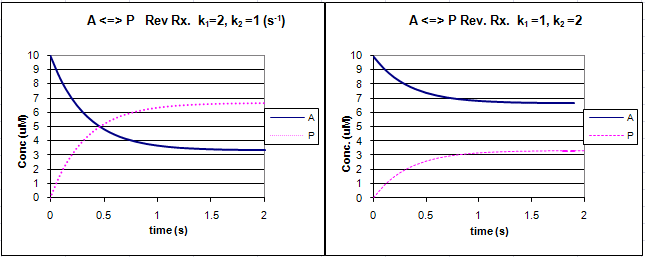
Graphs of A and P vs t for this reaction at two different sets of values of k1 and k2 are shown below.
Figure: Reversible First Order Reactions: A <=> P
 Xcel Spread Sheet: Reversible First Order Reactions -
Xcel Spread Sheet: Reversible First Order Reactions -
Go to the following spread sheet and change the values of k1 and k2. Note the changes in the graphs. Remember from our discussion of macromolecule:ligand binding, the dissociation constant, Kd, was related to the rate constants by the formula Kd = k2/k1. Note that if the first order rate constants for a reversible chemical reaction are equal, Keq (and its inverse) equal 1, and the equilibrium concentrations of A and P are equal.
![]() 4/26/13
4/26/13 Wolfram Mathematica CDF Player - Reversible First Order Reactions ([A] blue, [B] red) (free plugin required)
Wolfram Mathematica CDF Player - Reversible First Order Reactions ([A] blue, [B] red) (free plugin required)
Consecutive First Order Reactions
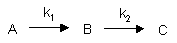
For these reactions:
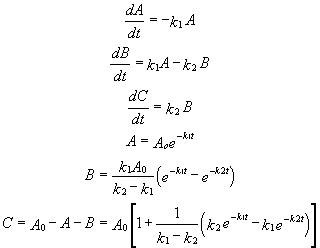
Graphs of A, B, and C vs t for these reaction at two different sets of values of k1 and k2 are shown below.
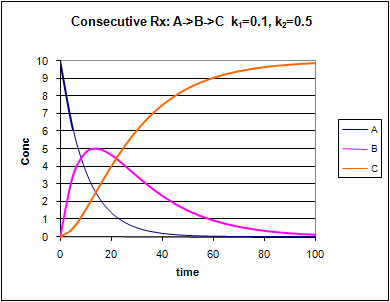
Figure: Consecutive Irreversible First Order Reactions: A --> B --> C
 Xcel Spread Sheet: Consecutive Reactions -
Xcel Spread Sheet: Consecutive Reactions -
Change the values of k1 and k2. Note the changes in the graphs.
![]() 4/26/13
4/26/13 Wolfram Mathematica CDF Player - Irreversible Consecutive First Order Reactions ([A] blue, [B] red, [C] orange (free plugin required)
Wolfram Mathematica CDF Player - Irreversible Consecutive First Order Reactions ([A] blue, [B] red, [C] orange (free plugin required)
![]() Reaction Appliets:
Reaction Appliets:
 Reactions Kinetics: Java Applet - Zero, First, and Second Order Reactions
Reactions Kinetics: Java Applet - Zero, First, and Second Order Reactions
 Consecutive reactions
Consecutive reactions
 Graphical determination of reaction order from initial rates
Graphical determination of reaction order from initial rates


