15.4B: Antibody-Antigen Binding
- Page ID
- 5430
Antibodies are proteins synthesized and secreted by B cells that bind to antigens. Most antigens are macromolecules: proteins, polysaccharides, even DNA and RNA. The interaction occurs by noncovalent forces (like that between enzymes and their substrate) between the antigen-combining site on the antibody and a portion of the antigen called the antigenic determinant or epitope.
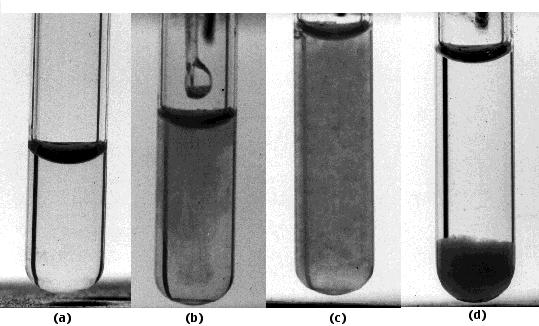
These photos show one type of interaction — precipitation — between antibodies and antigen.
- The tube contains antibodies to the Type III pneumococcal polysaccharide isolated from the capsule surrounding the bacteria.
- A solution of the polysaccharide is added.
- The formation of insoluble antigen-antibody complexes is revealed by the almost instantaneous appearance of turbidity.
- After an hour, the complexes settle out as a precipitate. If the proportion of antigen to antibody in the mixture is selected properly, the fluid above the precipitate will be devoid of both.
In the human body, this binding can literally be life-saving. The capsule that surrounds pneumococci protects them from phagocytosis. Pneumococci that fail to make a capsule — "R" forms — do not cause disease. If the appropriate antibodies are present in the body, they combine with the capsule. Coated with protein instead of polysaccharide, the pneumococci are now easy to ingest.
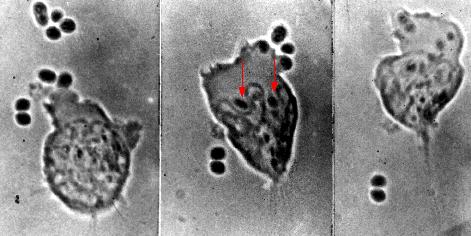
These photomicrographs show phagocytosis of antibody-coated pneumococci.
- Left: A neutrophil extends a pseudopod toward two pneumococci.
- Center: these bacteria have been engulfed (arrows), and the neutrophil is beginning to engulf four more pneumococci at the upper right.
- Right: Two pneumococci have escaped.
In the days before antibiotics, the start of antibody production by the immune system of the patient marked the turning point in the progression of the disease.
The Antigen-Combining Site
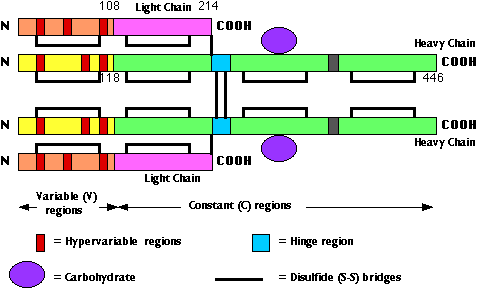
The fig. 15.4.2.3 shows the primary structure of an IgG antibody. Different IgG antibodies differ most markedly at the so-called hypervariable regions (shown in red):
- three in the heavy chain
- three in the light chain
In the three-dimensional (tertiary) structure of the molecule, the 6 hypervariable regions are brought close together and make up the antigen-combining site. For this reason, the hypervariable regions are also called complementarity determining regions (CDRs).
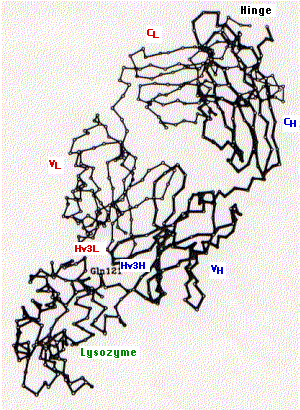
The fig. 15.4.2.4 shows the tertiary structure of one arm of an antibody specific for the lysozyme found in hen's egg white (upper right) bound to lysozyme (lower left).
The following are indicated on the diagram:
- constant region of the light chain (CL) and the first constant region of the heavy chain (CH)
- variable regions of the heavy (VH) and light chains (VL)
- the third hypervariable region of the heavy (Hv3H) and light (Hv3L) chains. The importance of the pair of third hypervariable or complementarity determining regions in binding the epitope is typical of both antibodies and of T cell receptors for antigen. This makes good sense as the opportunities for genetic diversity at those sites is far greater than for the first and second CDRs.
- Gln121 is a glutamine that is a dominant feature of the epitope on lysozyme
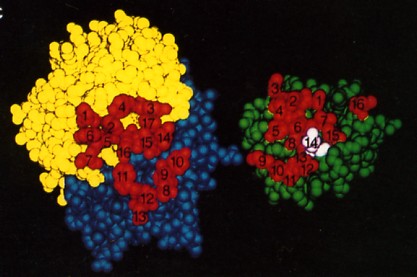
The fig. 15.4.2.5 is a space-filling model of the same antibody with the light chain in yellow, the heavy chain in blue. The view is looking down on the epitope-binding surface (left) and the epitope on lysozyme (right). In the antigen-antibody complex, the two surfaces fit snugly together. The 17 amino acid residues that contact lysozyme in the antigen-antibody complex are numbered (1–7 on the L chain, 8–17 on the H chain. The 16 amino acid residues of lysozyme that contact the antibody; that is, that make up its epitope are also numbered. Number 14 is Gln-121. The complementarity of the antigen-binding site and the epitope, their respective shapes and the opportunities for multiple noncovalent interactions determine how strongly the two bind together. The strength of the binding of an antibody to its antigen is called its affinity.


