B5. Proteins of the Neural Synapse
- Page ID
- 5593
We must now account for the rise and fall of the membrane potential to a variety of neurotransmitters, including the cholineric transmitters (ex. acetylcholine), catecholamines (dopamine, epinephrine, norepineprine), amino acid derivatives (ex. Glu, Asp, N-methyl-D-Asp, Gly, gamma-amino-butyric acid -GABA), and peptides (endorphins, encephalins). We will consider five membrane proteins:
Figure: five membrane proteins
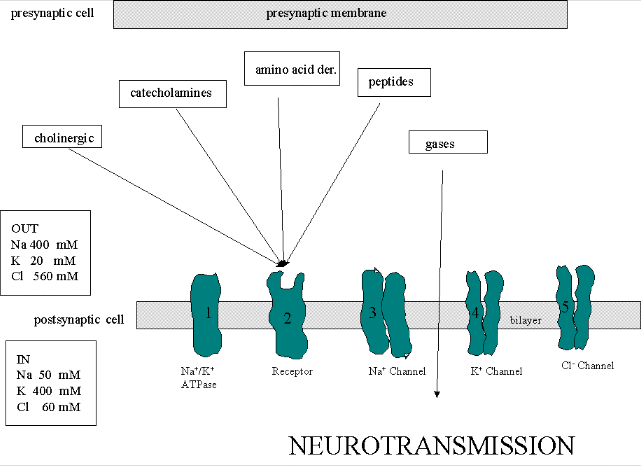
Na+-K+-ATPase: We discussed this in the previous section on active transport. It transports both sodium and potassium ions against a concentration gradient using ATP as an energy source. The protein is a sodium dependent ATPase. Without this protein, the membrane potential could not be maintained since the sodium and potassium gradient would collapse. It also contributes to the potential since its is an electrogenic antiporter. (In addition, we have seen that ungated potassium and sodium channels are also present.)
- Neurotransmitter receptor: The receptors we will consider here are typically ligand-gated ion channels. Once ligand binds, a conformational change occurs in the protein, allowing a flow of ions down a concentration gradient. Depending on the nature of the ion, the channel either initiates depolarization (when Na+ enters from the outside and raises ΔΨ) or inhibits depolarization (when Cl- enters from the outside and lowers ΔΨ). When chloride channels open, they hyperpolarize the transmembrane potential. Stimulatory neurotransmitters (like glutamate) lead to depolarization of the membrane, while inhibitory neurotransmitters (like gamma-aminobutyric acid) lead to hyperpolarization of the membrane (make the potential more negative).
- Na+ channel (voltage-gated): When the ligand-gated channel depolarizes the membrane to some threshold value, sodium channels undergo a conformational change and open allowing Na+ ions to flood into the cell, raising the potential to a positive approx. 33 mV (a value lower than the the equilibrium sodium potential). This membrane protein is a voltage-gated channel, not a ligand gated one. Somehow, it senses a change in the transmembrane potential initiated by opening of the ligand-gated channel.
- K+ channel (voltage gated): When the membrane potential reaches around +25 mV or so, the K+ channel, a voltage-gated membrane protein, alters its conformation, allowing K+ efflux from the cells, lowering the potential until it reaches the potassium equilibrium potential. It slowly relaxes back to the cell resting potential of about -60 mV. Recently, the crystal structure of the K+ channel from Streptomyces lividans was determined (Science, 280, 69-77, 1998): "All K+ channels show a selectivity sequence of K+, Rb+ > Cs+, whereas permeability for the smallest alkali metal ions Na+ and Li+ is immeasurably low. Potassium is at least 10,000 times more permeant than Na+, a feature that is essential to the function of K+ channels.....Two aspects of ion conduction by K+ channels have tantalized biophysicists for the past quarter century. First, what is the chemical basis of the impressive fidelity with which the channel distinguishes between K+ and Na+ ions, which are featureless spheres of Pauling radius 1.33 � and 0.95 �, respectively? Second, how can K+ channels be so highly selective and at the same time, apparently paradoxically, exhibit a throughput rate approaching the diffusion limit? The 104 margin by which K+ is selected over Na+ implies strong energetic interactions between K+ ions and the pore. And yet strong energetic interactions seem incongruent with throughput rates up to 108 ions per second."
- Cl- channel: If these channels (typically ligand-gated) are open, they will hyperpolarize the cell and make it more difficult to fire.
Contributors and Attributions


