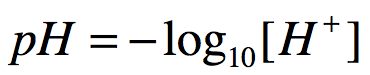W2017_Lecture_04_reading
- Page ID
- 7282
Chemical Reactions
Chemical reactions occur when two or more atoms bond together to form molecules or when bonded atoms are broken apart. The substances that "go in" to a chemical reaction are called the reactants (by convention these are usually listed on the left side of a chemical equation), and the substances found that "come out" of the reaction are known as the products (by convention these are usually found on the right side of a chemical equation). An arrow linking the reactants and products is typically drawn between them to indicate the direction of the chemical reaction. By convention, for one-way reactions, reactants are listed on the left and products on the right of the single-headed arrow. However, you should be able to identify reactants and products of one-way reactions that are written in any orientation (e.g. right-to-left; top-to-bottom, diagonal right-to-left, around a circular arrow, etc.)
\[\underbrace{2H_2O_2}_{\text{hydrogen peroxide}} → \underbrace{2H_2O}_{\text{water}} + \underbrace{O_2}_{\text{oxygen}}\]
Note:
Practice: Identify the reactants and products of the reaction involving hydrogen peroxide above.
Note:
Possible discussion: When we write \(H_2O_2\) to represent the molecule hydrogen peroxide it is a model representing an actual molecule. What information about the molecule is immediately communicated by this molecular formula? That is, what do you know about the molecule just by looking at the term \(H_2O_2\)?
What information is not explicitly communicated about this molecule by looking only at the formula?
Some chemical reactions, such as the one shown above, proceed mostly in one direction. When we depict reactions with a single-headed (unidirectional) arrow we are implying that the reaction is essentially irreversible. However, all reactions can technically proceed in both directions. Reversible reactions are those that can proceed in either direction. In reversible reactions, reactants are turned into products, but when the concentration of product goes beyond a certain threshold (a characteristic particular to a specific reaction), some of these products will be converted back into reactants. This back and forth continues until a certain relative balance between reactants and products occurs—a state called equilibrium. These situations of reversible reactions are often denoted by a chemical equation with a double headed arrow pointing towards both the reactants and products. You will find a continuum of chemical reactions, some proceed mostly in one direction and nearly never reverse, while others change direction easily depending on various factors like the relative concentrations of reactants and products. That is, you will find reactions with all sorts of equilibrium points.
Note:
Use of vocabulary: You may have realized that the terms "reactants" and "products" are relative to the direction of the reaction. If you have a reaction that is reversible, though, the products of running the reaction in one direction become the reactants of the reverse. You can label the same compound with two different terms. That can be a bit confusing. So, what is one to do in such cases? The answer is that if you want to use the terms "reactants" and "products" you must be clear about the direction of reaction that you are referring to.
Let's look at an example of a reversible reaction in biology. In human blood, excess hydrogen ions (H+) bind to bicarbonate ions (HCO3-) forming an equilibrium state with carbonic acid (H2CO3). This reaction is readily reversible. If carbonic acid were added to this system, some of it would be converted to bicarbonate and hydrogen ions as the chemical system sought out equilibrium.
\[HCO_3^−+ H^+ \rightleftharpoons H_2CO_3 \label{2}\]
The examples above examine "idealized" chemical systems as they might occur in a test-tube. In biological systems, however, equilibrium for a single reaction is rarely obtained as it might be in the lab. In biological systems reactions do not occur in isolation. The concentrations of the reactants and/or products are constantly changing, often with a product of one reaction being a reactant for another reaction. These linked reactions form what are known as biochemical pathways. The immediate example above illustrates this and another caveat. While the reaction between the bicarbonate/proton and carbonic acid is highly reversible, it turns out that physiologically this reaction is usually "pulled" towards the formation of carbonic acid. Why? As shown below, carbonic acid becomes a reactant for another biochemical reaction, its conversion to CO2 and H2O. This conversion reduces the concentration of H2CO3 thus pulling the reaction between bicarbonate and H+ to the right. Moreover, a third reaction, the removal of CO2 and H2 from the system also pull the reaction further to the right. These kinds of reactions are important contributors to maintaining the H+ homeostasis of our blood.
What is the role of Acid/Base Chemistry in Bis2A?
We have learned that the behavior of chemical functional groups depend greatly on the composition, order and properties of their constituent atoms. As we will see, some of the properties of key biological functional groups can be altered depending on the pH (hydrogen ion concentration) of the solution that they are bathed in. For example, some of the functional groups on the amino acid molecules that make up proteins can exist in different chemical states depending on the pH. We will learn that the chemical state of these functional groups in the context of a protein can have have a profound effect on the shape of protein or its ability to carry out chemical reactions. As we move through the course we will see numerous examples of this type of chemistry in different contexts.
Defining pH
pH is formally defined as:
In the equation above, the square brackets surrounding H+ indicate concentration.
Attribution: Marc T. Facciotti (own work)
If necessary try a math review at wiki logarithm or kahn logarithm.
Also see: concentration dictionary or wiki concentration.
Hydrogen ions are spontaneously generated in pure water by the dissociation (ionization) of a small percentage of water molecules into equal numbers of hydrogen (H+) ions and hydroxide (OH-) ions. While the hydroxide ions are kept in solution by their hydrogen bonding with other water molecules, the hydrogen ions, consisting of naked protons, are immediately attracted to un-ionized water molecules, forming hydronium ions (H30+). Still, by convention, scientists refer to hydrogen ions and their concentration as if they were free in this state in liquid water. This is another example of a shortcut that we often take - it's easier to write H+ rather than H3O+. We just need to realize that this shortcut is being taken, else confusion will ensue.
pH of a solution is a measure of the concentration of hydrogen ions in a solution (or the number of hydronium ions). The number of hydrogen ions is a direct measure of how acidic or how basic a solution is. The pH scale is logarithmic and ranges from 0 to 14 (Figure 2). We define pH=7.0 as neutral. Anything with a pH below 7.0 is termed acidic and any reported pH above 7.0 is termed alkaline or basic. Extremes in pH in either direction from 7.0 are usually considered inhospitable to life, though examples exist to the contrary. pH in the human body usually ranges between 6.8 and 7.4, except in the stomach where the pH is typically between 1 and 2.
Watch this video for a straightforward explanation of pH and its logarithmic scale.
The pH scale ranging from acidic to basic with various biological compounds or substances that exist at that particular pH.
Attribution: Marc T. Facciotti (own work)
Watch this video for an alternative explanation of pH and its logarithmic scale.
The concentration of hydrogen ions dissociating from pure water is 1 × 10-7 moles H+ ions per liter of water. One Mole (mol) of a substance (which can be atoms, molecules, ions, etc), is defined as being equal to 6.02 x 1023 particles of the substance. Therefore, 1 mole of water is equal to 6.02 x 1023 water molecules. The pH is calculated as the negative of the base 10 logarithm of this unit of concentration. The log10 of 1 × 10-7 is -7.0, and the negative of this number yields a pH of 7.0, which is also known as neutral pH.
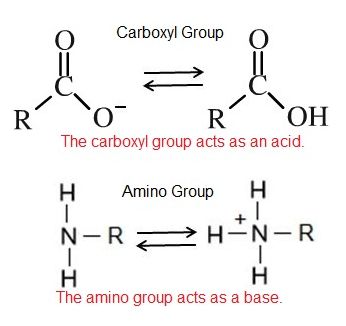
Non-neutral pH readings result from dissolving acids or bases in water. High concentrations of hydrogen ions yields a low pH number, whereas low levels of hydrogen ions result in a high pH. This inverse relationship between pH and the concentration of protons confuses many students - take the time to convince yourself that you "get it". An acid is a substance that increases the concentration of hydrogen ions (H+) in a solution, usually by having one of its hydrogen atoms dissociate. For example, we have learned that the carboxyl functional group is an acid. The hydrogen atom can dissociate from the oxygen atom resulting in a free proton and a negatively charged functional group. A base provides either hydroxide ions (OH–) or other negatively charged ions that combine with hydrogen ions, effectively reducing the H+ concentration in the solution and thereby raising the pH. In cases where the base releases hydroxide ions, these ions bind to free hydrogen ions, generating new water molecules. For example, we have learned that the amine functional group is a base. The nitrogen atom will accept hydrogen ions in solution, thereby reducing the number of hydrogen ions which raises the pH of the solution.
Additional pH resources
Here are some additional links on pH and pKa to help learn the material. Note that there is an additional module devoted to pKa.
Chemwiki Links
Khan Academy Links
Simulations
pKa
pKa is defined as the negative log10 of the dissociation constant of an acid, its Ka. Therefore, the pKa is a quantitative measure of how easily or how readily the acid gives up its proton [H+] in solution and thus a measure of the "strength" of the acid. Strong acids have a small pKa, weak acids have a larger pKa.
The most common acid we will talk about in Bis2A is the carboxylic acid functional group. These acids are typically weak acids, meaning that they only partially dissociate (into H+ cations and RCOO- anions) in neutral solution. HCL (hydrogen chloride) is a common strong acid, meaning that it will fully dissociate into H+ and Cl-.
Note that the key difference in the figure below between a strong acid or base and a weak acid or base is the single arrow (strong) versus a double arrow (weak). In the case of the single arrow you can interpret that by imagining that nearly all reactants have been converted into products. Moreover, it is difficult for the reaction to reverse backwards to a state where the protons are again associated with the molecule there were associated with before. In the case of a a weak acid or base the double-sided arrow can be interpreted by picturing a reaction that can:
(a) have both forms of the conjugate acid or base (that is what we call the molecule that "holds" the proton - i.e. CH3OOH and CH3OO-, respectively in the figure) present at the same time and
(b) that the ratio of those two quantities can change easily by moving the reaction in either direction.
An example of strong acids and strong bases in their protonation and deprotonation states. The value of their pKa is shown on the left.
Attribution: Marc T. Facciotti (own work)
Electronegativity plays a role in the strength of an acid. If we consider the hydroxyl group as an example, the greater electronegativity of the atom or atoms (indicated R) attached to the hydroxyl group in the acid R-O-H results in a weaker H-O bond, which is thus more readily ionized. This means that the pull on the electrons away from the hydrogen atom gets greater when the oxygen atom attached to the hydrogen atom is also attached to another electronegative atom. An example of this is HOCL. The electronegative Cl polarizes the H-O bond, weakening it and facilitating the ionization of the hydrogen. If we compare this to a weak acid where the oxygen is bound to a carbon atom (as in carboxylic acids) the oxygen is bound to the hydrogen and carbon atom. In this case, the oxygen is not bound to another electronegative atom. Thus the H-O bond is not further destabilized and the acid is considered a weak acid (it does not give up the proton as easily as a strong acid).
The strength of the acid can be determined by the electronegativity of the atom the oxygen is bound to. For example, the weak acid Acetic Acid, the oxygen is bound to carbon, an atom with low electronegativity. In the strong acid, Hypochlorous acid, the oxygen atom is bound to an even more electronegative Chloride atom.
Attribution: Erin Easlon (own work)
In Bis2A you are going to be asked to relate pH and pKa to each other when discussing the protonation state of an acid or base, for example, in amino acids. How can we use the information given in this module to answer the question: Will the functional groups on the amino acid Glutamate be protonated or deprotonated at a pH of 2, at a pH of 8, at a pH of 11?
In order to start answering this question we need to create a relationship between pH and pKa. The relationship between pKa and pH is mathematically represented by Henderson-Hasselbach equation shown below, where [A-] represents the deprotonated form of the acid and [HA] represents the protonated form of the acid.
The Henderson-Hasselbach equation.
A solution to this equation is obtained by setting pH = pKa. In this case, log([A-] / [HA]) = 0, and [A-] / [HA] = 1. This means that when the pH is equal to the pKa there are equal amounts of protonated and deprotonated forms of the acid. For example, if the pKa of the acid is 4.75, at a pH of 4.75 that acid will exist as 50% protonated and 50% deprotonated. This also means that as the pH rises, more of the acid will be converted into the deprotonated state and at some point the pH will be so high that the majority of the acid will exist in the deprotonated state.
This graph depicts the protonation state of acetic acid as the pH changes. At a pH below the pKa, the acid is protonated. At a pH above the pKa the acid is deprotonated. If the pH equals the pKa, the acid is 50% protonated and 50% deprotonated.
Attribution: Ivy Jose (own work)
In Bis2A we will be looking at the protonation state and deprotonation state of amino acids. Amino acids contain multiple functional groups that can be acids or bases. Therefore their protonation/deprotonation status can be more complicated. Below is the relationship between the pH and pKa of the amino acid Glutamic Acid. In this graph we can ask the question we posed earlier: Will the functional groups on the amino acid Glutamate be protonated or deprotonated at a pH of 2, at a pH of 8, at a pH of 11?
This graph depicts the protonation state of glutamate as the pH changes. At a pH below the pKa for each functional group on the amino acid, the functional group is protonated. At a pH above the pKa for the functional group it is deprotonated. If the pH equals the pKa, the functional group is 50% protonated and 50% deprotonated.
Attribution: Ivy Jose (own work)
Note: Possible discussion
- What is the overall charge of free Glutamate at a pH of 5?
- What is the overall charge of free Glutamate at a pH of 10?