Investigation: Observing Diffusion and Semi-Permeable Membranes
- Page ID
- 26903
This page is a draft and is under active development.
How Can Diffusion Be Observed?
Introduction: In this lab you will observe the diffusion of a substance across a semi permeable membrane. Iodine is a known indicator for starch. An indicator is a substance that changes color in the presence of the substance it indicates. Watch as your teacher demonstrates how iodine changes in the presence of starch.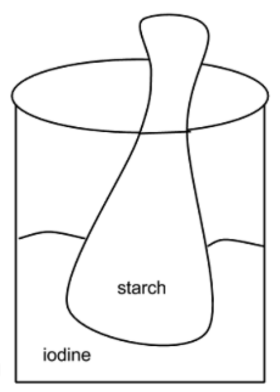
Prelab Observations: Describe what happened when iodine came into contact with starch.
Procedure:
1. Fill a plastic baggie with a teaspoon of corn starch and a half a cup of water tie bag. (This may already have been done for you)
2. Fill a beaker halfway with water and add ten drops of iodine.
3. Place the baggie in the cup so that the cornstarch mixture is submerged in the iodine water mixture.
4. Wait fifteen minutes and record your observations in the data table
5. While you are waiting, answer the questions.
Questions:
1. Define diffusion. ____________________________________________________________
2. Define osmosis. ____________________________________________________________
3. Why is iodine called an indicator? _____________________________________________________
4. Molecules tend to move from areas of _______ concentration to areas of ______ concentration.
What's in the Bag?
We're going to think about concentrations now, which substances are more or less concentrated depends on which one has the most stuff in it.
1. Which is more concentrated in starch? [ beaker / baggie ]
2. Which is more concentrated in iodine? [ beaker / baggie ]
3. With regard to iodine, which is hypertonic? [ beaker / baggie ]
4. With regard to starch, which is hypertonic? [ beaker / baggie ]
Make Some Predictions
1. If the bag is permeable to starch, which way would the starch move? [ into bag / out of bag ]
2. If the bag is permeable to iodine, which way would the iodine move? [ into bag / out of bag ]
3. If the bag is permeable to iodine, what color would you expect it to change? [ orange / purple / no change ]
What about the solution in the beaker? [ orange / purple / no change ]
4. If the bag is permeable to starch, what color would you expect it to change? [ orange / purple / no change ]
What about the solution in the beaker? [ orange / purple / no change ]
Observations
Write your observations in the table below:
| Starting Color | Color after 15 minutes | |
| Solution in Beaker | ||
| Solution in Bag |
Post Lab Analysis
1. Based on your observations, which substance moved, the iodine or the starch?
2. How did you determine this?
3. The plastic baggie was permeable to which substance?
4. Explain how the bag is a model for the cell.
5. Sketch the cup and baggie in the space below. Use arrows to illustrate how diffusion occurred in this lab.
6. What would happen if you did an experiment in which the iodine solution was placed in the baggie, and the starch solution was in the beaker? Be detailed in your description.
7. Why is it not a good idea to store iodine in a plastic bag? Related Documents:

