5.2: Procedures
- Page ID
- 15979
Make sure you follow aseptic procedures and label everything carefully! Use the inoculation method indicated for each type of medium—these methods may differ. Make sure to thoroughly sterilize your loop or needle between inoculations to ensure that you are only introducing one bacterial species into your medium. If a medium is inoculated with more than one kind of bacterium, a positive result cannot be attributed to a single bacterial species. Be sure to use the correct bacterial species for each test. Follow directions carefully so that you do not waste media.
For comparison purposes, you will be provided with negative controls (media that have not been inoculated) in the next lab when you are analyzing your results.
Note
All media that you inoculate today will be incubated until the next lab, when you will analyze your results .
A. Carbohydrate Fermentation
Each student: 1 tube each of the following broths: Lactose + phenol red (green cap), Sucrose + phenol red (yellow cap), Glucose + phenol red (red cap)
Instructions: Choose 1 of the following bacteria: Proteus vulgaris, Escherichia coli, Bacillis subtilis, or Streptococcus faecalis (each person at the table should choose a different species)
Inoculate the 3 types of fermentation broth with your chosen bacteria. Prior to inoculating the broths, make note of any small bubbles that might be present in the Durham tubes, so these are not read as evidence of gas formation during fermentation.
B. Gelatin Hydrolysis
Each student: 1 nutrient gelatin deep
Instructions: Choose 1 of the following bacteria: Staphylococcus aureus, Serratia marcescens, Streptococcus faecalis, or Bacillis subtili
Use an inoculation needle (not a loop) to obtain some bacteria from your stock culture. Inoculate the gelatin deep by using the inoculating needle to stab ~3/4 of the way down into the nutrient gelatin and then drawing the needle straight back up.
C. Starch Hydrolysis
Each student: 1 starch agar plate
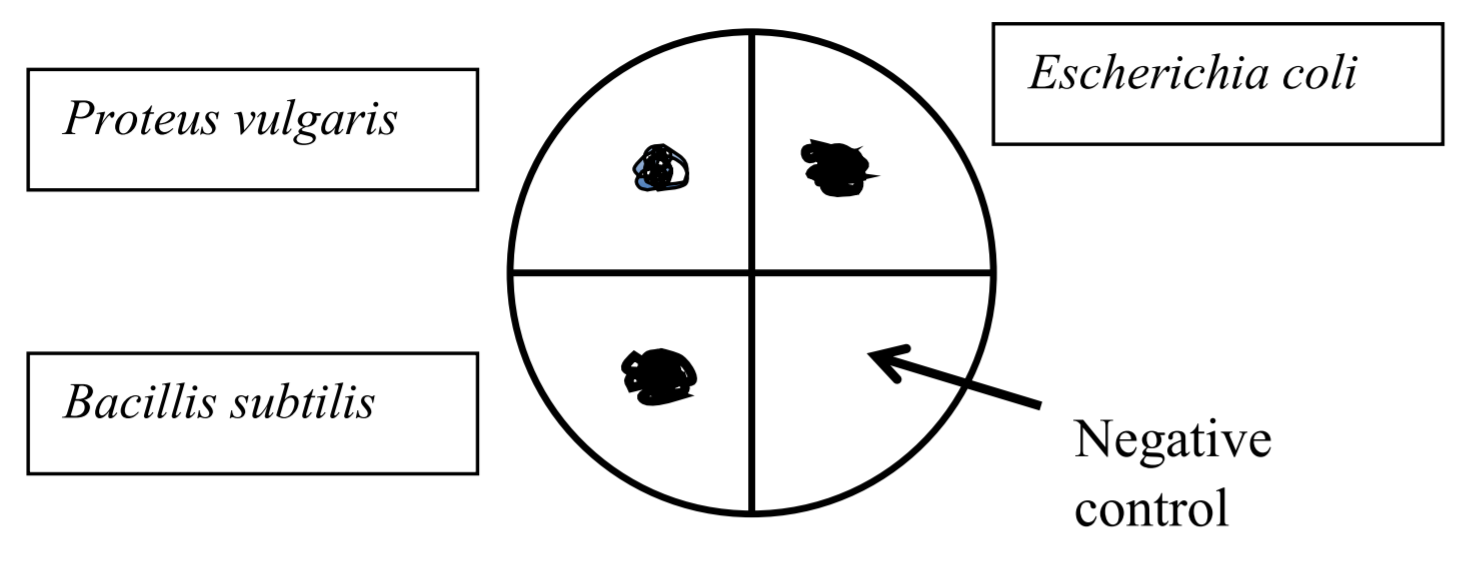
Instructions: Use all of the following bacteria: Proteus vulgaris, Escherichia coli, Bacillus subtilis
Divide your starch agar plate into 4 areas using a wax pencil or a sharpie marker. Make sure you draw on the bottom of the petri dish (the part that contains the agar) rather than on the lid. Label the plate to indicate which bacterium will be inoculated into each area. One area is left as a negative control.
Using a loop, you will do spot inoculations of each bacterial species in the areas. A spot inoculation is used to ensure that a large amount of bacteria will grow at a single location and produce a concentrated amount of amylase. The spot inoculation technique is shown below.
.png?revision=1&size=bestfit&width=575&height=227)
D. Casein Hydrolysis
Each student: 1 milk agar plate
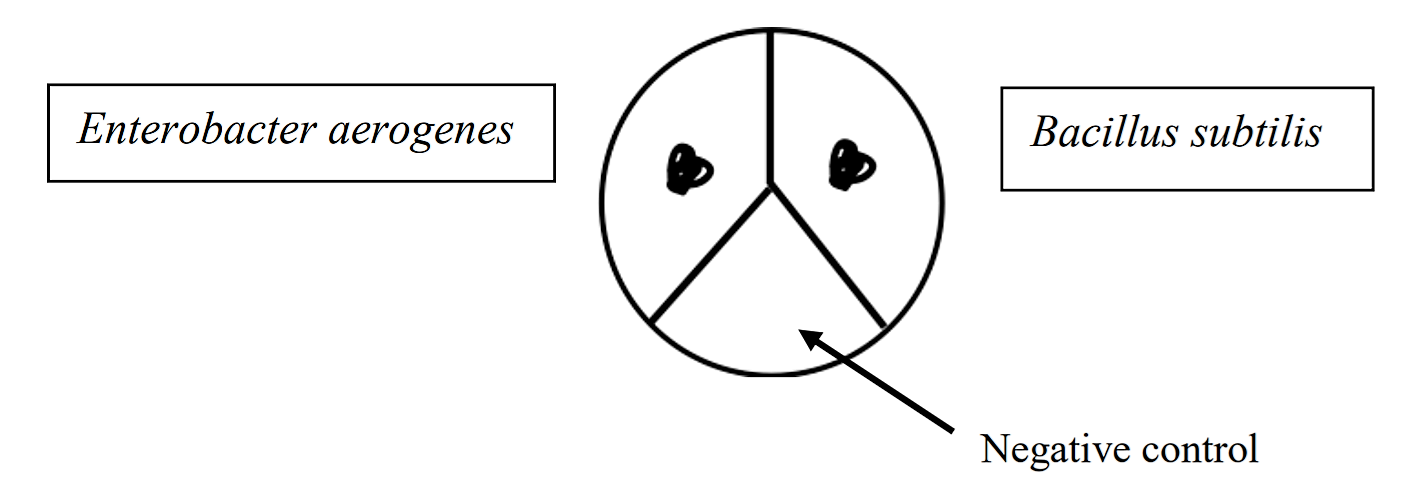
Instructions: Use both these bacteria: Bacillus subtilis and Enterobacter aerogenes
Divide your milk agar plate into 3 areas using a wax pencil or a sharpie marker. Label the plate to indicate which bacterium will be inoculated into each area. One area is left as a negative control. Using a loop, you will do spot inoculations of each bacterial species in the areas, just as you did on the starch agar plate.
.png?revision=1&size=bestfit&width=577&height=205)
E. Urea Hydrolysis
Each pair of students: 1 urea slant
Instructions: Choose one of these bacteria: Escherichia coli or Proteus vulgaris
To inoculate the urea slant, use a loop to obtain some bacteria from the culture, and then carefully streak the surface of the slant. Do not stab down into the slant’s interior. Replace the cap on the slant, but leave the cap somewhat loose. Wrap in the tube in aluminum foil.
F. Citrate Utilization
Each student: 1 Simmon’s citrate slant
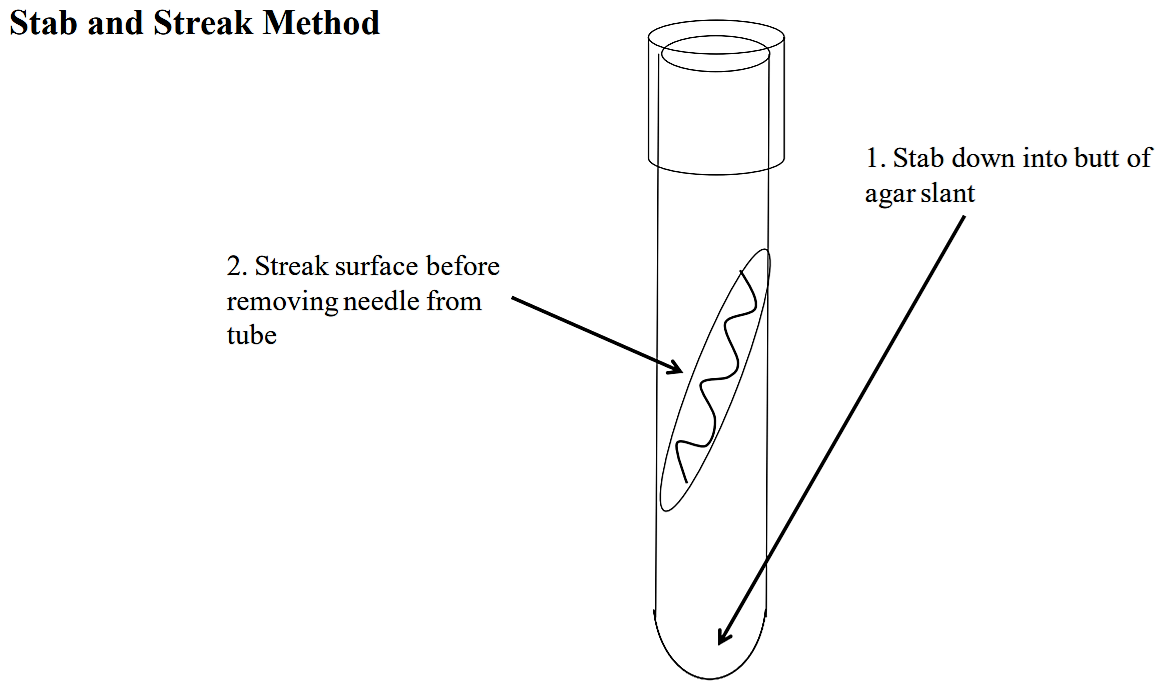
Instructions: Choose one of these bacteria: Escherichia coli or Serratia marcescens
Using an inoculation needle (not a loop), inoculate the Simmon’s citrate by first stabbing the needle into the butt of the agar slant (see below) and then streaking the surface of the slant before you pull the needle out of the tube.
.png?revision=2&size=bestfit&width=585&height=344)
G. Catalase Activity
Each student: 1 TSA slant
Instructions: Choose one of these bacteria: Staphylococcus aureus or Streptococcus faecalis
Using a loop, inoculate the surface of the TSA slant. Make sure to use a heavy inoculum.
H. Tryptophan Hydrolysis
Each student: 1 tryptone broth (Note: this medium contains tryptophan)
Instructions: Choose one of these bacteria: Escherichia coli or Enterobacter aerogenes
Inoculate the medium with your chosen bacteria. Make sure to use a heavy inoculum.


