1.1: Introduction and Background
- Page ID
- 25801
An Introduction and Background for URIECA Modules 4 and 5
Abl and Bcr-Abl Proteins
Abelson (c-Abl or Abl) is a protein tyrosine kinase that is involved in a number of highly-regulated cellular processes, including cell division, differentiation and adhesion. In healthy cells, c-Abl is auto-inhibited by domains at its amino (N)-terminus. This means that the kinase activity of c-Abl is tightly regulated, and the default activity setting is “off”.
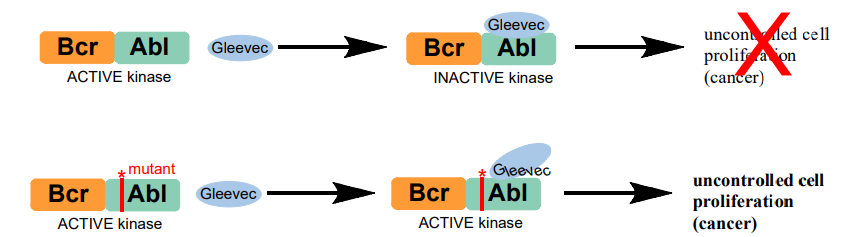
A chromosomal abnormality implicated in chronic myleloid leukemia (CML) causes the reciprocal translocation of genetic material from two different chromosomes, 9 and 22, which results in the formation of a mutant gene that contains part of the BCR (break cluster region) gene from chromosome 22 and part of the ABL gene from chromosome 9. This mutant gene is called BCR-ABL, and the protein it encodes, denoted Bcr-Abl, contains the kinase domain of c-Abl, but lacks the residues responsible for autoinhibition. Bcr-Abl is therefore a constitutively active kinase, which means that the enzyme activity is permanently “on”. This aberrant kinase activity is responsible for uncontrolled cell proliferation, which leads to cancer.

A Small Molecule Drug for CML Treatment
Bcr-Abl activity is the underlying cause for CML, and the identification of the Bcr-Abl oncoprotein led to high throughput screening and the “rational design” of potential small molecule inhibitors. These efforts culminated in the development of the drug Gleevec by chemists at the pharmaceutical company Novartis (a branch of which is a few doors down from us on Mass Ave). Gleevec (also known as Glivec, imatinib, and STI-571) showed excellent efficacy against CML and was approved by the FDA in 2001. Gleevec inhibits Bcr-Abl tyrosine kinase activity by competitively binding in the ATP binding pocket of the kinase domain and stabilizing the inactive conformation of the protein. This development was particularly thrilling to the scientific community because Gleevec is the first example of a small molecule tyrosine kinase inhibitor to treat human disease. Also exciting is the striking specificity of Gleevec for Abl. Gleevec only inhibits two other proteins at physiological levels, neither of which result in problematic side effects.

However….A PERCENTAGE OF CML PATIENTS DO NOT RESPOND TO GLEEVEC TREATMENT, AND OTHER PATIENTS THAT INITIALLY RESPOND TO TREATMENT EVENTUALLY DEVELOP GLEEVEC RESISTANCE. ontrolled liferation X cell pro (cancer)
The majority of these Gleevec-resistant cases can be linked to a single amino acid mutation in the Abl kinase domain of the Bcr-Abl protein. Over 30 different point mutations have been identified in Gleevec-resistant CML patients (see appendix B3).
The Abl kinase domain is often used as a model for the full-length Bcr-Abl protein. The Abl kinase domain, similar to Bcr-Abl, lacks the N-terminal Abl regulation domains and is thus constitutively active. During this course you will express and purify an H396P mutant of the Abl kinase domain, a mutation that has been identified in patients with Gleevec-resistent CML. You will use this mutant along with (commercially available) wild-type Abl kinase domain in a coupled phosphorylation assay to determine kinase activity in the absence and presence of Gleevec and another kinase inhibitor, Dasatinib. In addition, you will use site-directed mutagenesis to create a DNA expression vector for future expression of an another Gleevec-resistant Abl mutant of your choice.



