8.1: Carbohydrates overview
- Page ID
- 66017
Carbohydrates
Carbohydrates are one of the four main classes of macromolecules that make up all cells and are an essential part of our diet; grains, fruits, and vegetables are all natural sources. While we may be most familiar with the role carbohydrates play in nutrition, they also have a variety of other essential functions in humans, animals, plants, and bacteria. In this section, we will discuss and review basic concepts of carbohydrate structure and nomenclature, and a variety of functions they play in cells.
Molecular structures
In their simplest form, carbohydrates can be represented by the stoichiometric formula (CH2O
Nomenclature
One issue with carbohydrate chemistry is the nomenclature. Here are a few quick and simple rules:
- Simple carbohydrates, such as glucose, lactose, or dextrose, end with an "-ose."
Simple carbohydrates can be classified based on the number of carbon atoms in the molecule, as with triose (three carbons),pentose (five carbons), or hexose (six carbons).Simple carbohydrates can be classified based on the functional group found in the molecule,i.e ketose (contains a ketone) or aldose (contains an aldehyde).- Polysaccharides
are often organized by the number of sugar molecules in the chain, such as in a monosaccharide, disaccharide, or trisaccharide.
For a short video on carbohydrate classification, see the 10-minute Khan Academy video by clicking here.
Monosaccharides
Monosaccharides ("mono-" = one; "

Figure 1.
Glucose versus galactose
Galactose (part of
Fructose versus both glucose and galactose
In glucose and galactose, the carbonyl group is on the C1 carbon, forming an aldehyde group. In fructose, the carbonyl group is on the C2 carbon, forming a ketone group. The former sugars
Figure 2. Glucose, galactose, and fructose are all hexoses. They are structural isomers, meaning they have the same chemical formula (C6H12O6) but a different arrangement of atoms.
Linear versus ring form of the monosaccharides
Monosaccharides can exist as a linear chain or as ring-shaped molecules. In aqueous solutions, monosaccharides are usually found in ring form (Figure 3). Glucose in a ring form can have two different arrangements of the hydroxyl group (OH) around the anomeric carbon (C1 that becomes asymmetric in the process of ring formation). If the hydroxyl group is below C1 in the sugar,
Figure 3. Five- and six-carbon monosaccharides exist in equilibrium between linear and ring form. When the ring forms, the side chain it closes on
Disaccharides
Disaccharides ("di-" = two) form when two monosaccharides undergo a dehydration reaction (also known as a condensation reaction or dehydration synthesis). During this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another monosaccharide, releasing a molecule of water and forming a covalent bond. A covalent bond formed between a carbohydrate molecule and another molecule (in this case, between two monosaccharides) is known as a glycosidic bond. Glycosidic bonds (also called glycosidic linkages) can be of the alpha or the beta type.
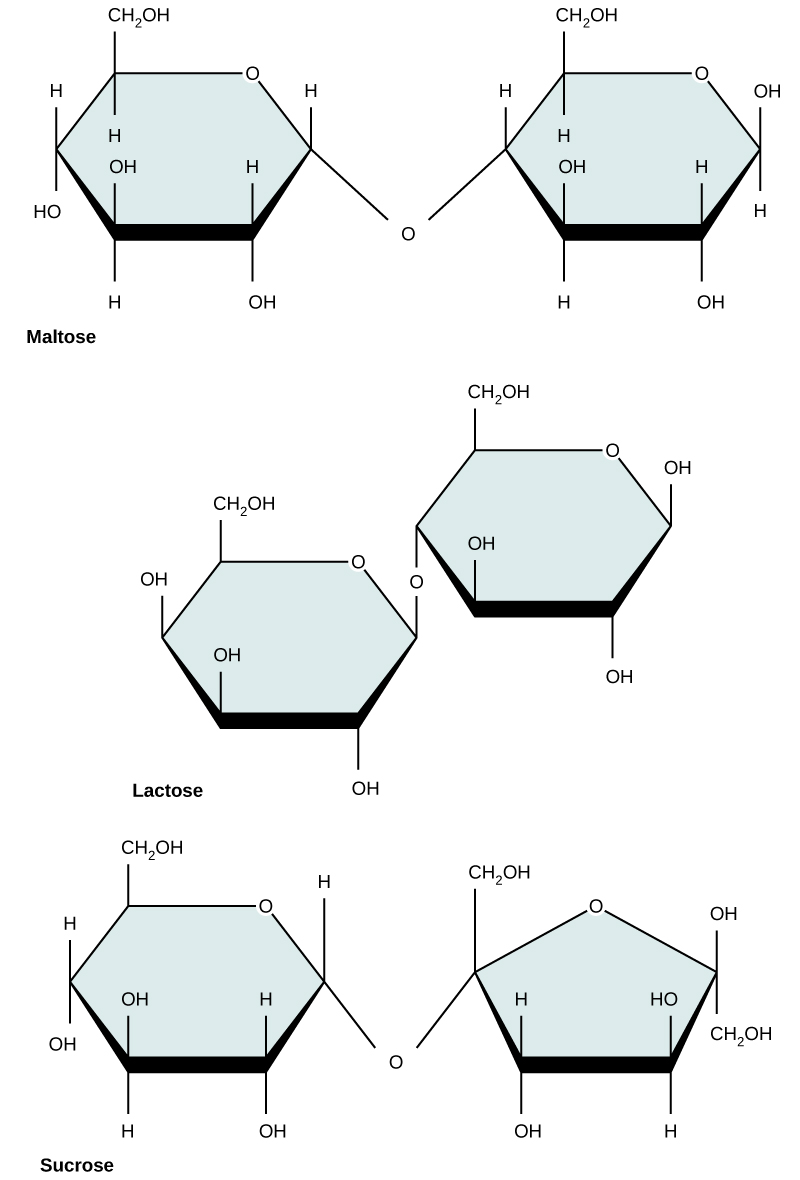
Figure 4. Sucrose
Common disaccharides include lactose, maltose, and sucrose (Figure 5). Lactose is a disaccharide
Figure 5. Common disaccharides include maltose (grain sugar), lactose (milk sugar), and sucrose (table sugar).
| Sucrose | Lactose | Maltose |
Polysaccharides
A long chain of monosaccharides linked by glycosidic bonds is known as a polysaccharide ("poly-" = many). The chain may
Starch is the stored form of
Figure 6. Amylose and amylopectin are two different
Glycogen
Glycogen is a common stored form of glucose in humans and other vertebrates. Glycogen is the animal equivalent of starch and is a highly branched molecule usually stored in liver and muscle cells. Whenever blood glucose levels decrease,
| Glycogen |
Cellulose
Cellulose is the most abundant natural biopolymer.
Figure 7. In cellulose,
As shown in the figure above, every other glucose monomer in cellulose
Interactions with carbohydrates
We have just discussed the various types and structures of carbohydrates found in biology. The next thing to address is how these compounds interact with other compounds. The answer to that is that it depends on the final structure of the carbohydrate. Because carbohydrates have many hydroxyl groups associated with the molecule, they are therefore excellent H-bond donors and acceptors. Monosaccharides can quickly and easily form H-bonds with water and are readily soluble. All of those H-bonds also make them quite "sticky". This is also true for many disaccharides and many short-chain polymers. Longer polymers may not be readily soluble.
Finally, the ability to form a variety of H-bonds allows polymers of carbohydrates or polysaccharides to form strong intramolecular and
Possible NB Discussion  Point
Point
Lipids and carbohydrates are not just classes of macromolecules that we discuss in BIS 2A but are also two of the essential macronutrients that we can obtain from eating various foods. Some popularized diet programs (e.g., Atkins, ketogenic) suggest limiting carbohydrates and/or fats. As you learn more about biomolecules and their roles in living systems, are you refining your perspective on foods and diets? What have you learned so far? Do you think there is anything missing in your understanding? Are you able to better understand and evaluate certain diets, such as the aforementioned?