8.6: Stratospheric Ozone Depletion
- Page ID
- 69453
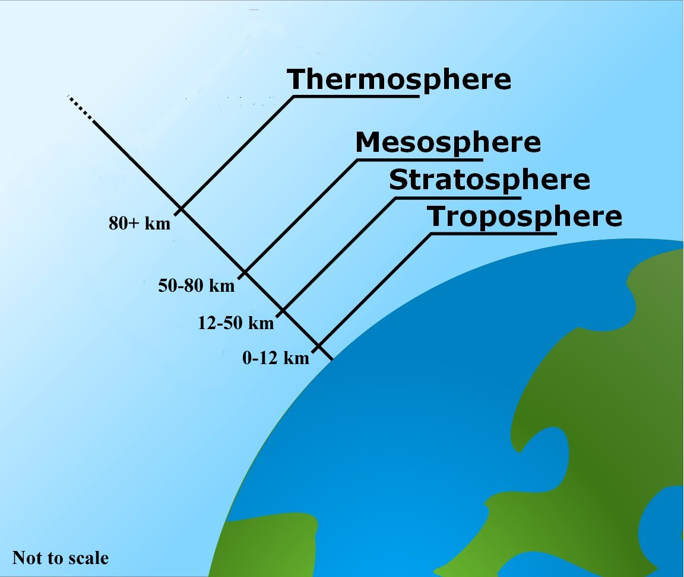
Figure \(\PageIndex{b}\): The layers of the atmosphere. the toposphere is the closest to the Earth's surface (0-12 km). Next, is the stratosphere (12-50 km), mesophere (50-80 km), and thermosphere (80+ km). The outermost layer (the exosphere) is not shown. Ground-level ozone in the troposphere is a form of air pollution, but the ozone layer in the stratosphere helps filter UV rays. Image by GFDL (CC-BY-SA).
In contrast to ground-level ozone, ozone in the stratosphere (which begins about 7 miles [11 km] up — where airliners cruise) protects life on Earth from the damaging effects of solar ultraviolet (UV) radiation (figure \(\PageIndex{1}\)). The concentration of ozone high in the stratosphere has declined over the past two decades . Satellite monitoring of the stratosphere, which began in 1978, has revealed a marked decline. The most serious decline occurs over Antarctica in spring (October) when a precipitous drop in ozone causes an ozone hole (figure \(\PageIndex{2}\)). The Dobson unit is a measure of the number of molecules of ozone in a vertical column of the atmosphere.
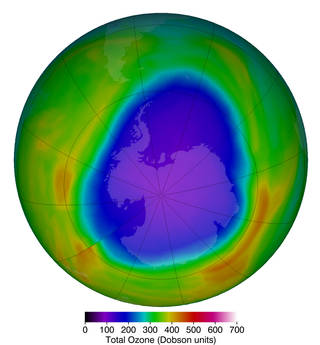
Figure \(\PageIndex{2}\): The Antarctic ozone hole that occurs annually in September and October during the Southern Hemisphere spring typically sees much lower ozone levels in than the Arctic. The purples and deep blues show the extent of low ozone levels on October 12, 2018, when they dropped to 104 Dobson units. Image and caption by NASA's Goddard Space Flight Center (public domain).
The ozone depletion process begins when CFCs (chlorofluorocarbons) and other ozone-depleting substances (ODS) are emitted into the atmosphere (figure \(\PageIndex{3}\)). After a period of several years, ODS molecules reach the stratosphere, about 10 kilometers above the Earth’s surface (figure \(\PageIndex{1}\)). CFCs are synthetic gases in which the hydrogen atoms of methane are replaced by atoms of fluorine and chlorine (e.g., CHF2Cl, CFCl3, CF2Cl2). These gases are noninflammable, nontoxic, and very stable. They were widely used in industry as refrigerants (e.g., in refrigerators and air conditioners), solvents, propellants in aerosol cans (now banned in some countries), and in the manufacture of plastic foams. CFCs escape to the air from all of these uses (e.g., from leaky and discarded refrigeration units). Their chemical inertness, which makes CFCs so desirable for industry, also makes them a threat to the atmosphere. Once in the atmosphere, it may take 60–100 years for them to decompose and disappear.
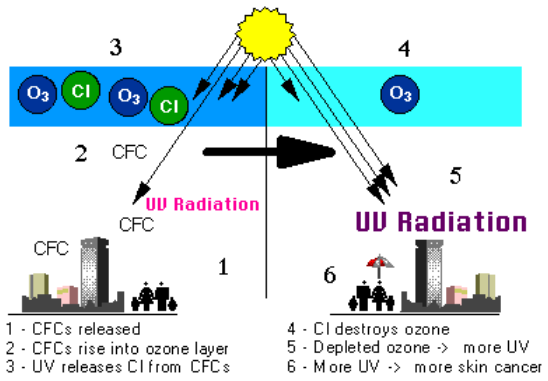
Figure \(\PageIndex{3}\): Strong ultraviolet (UV) light breaks apart the ozone-depleting substances (ODS). The process is as follows: (1) chlorofluorocarbons (CFCs) released, (2) CFCs rise into ozone layer in the stratosphere, which contains ozone (O3), (3) UV radiation releases chlorine (Cl) from CFCs, (4) Cl destroys ozone, (5) depleted ozone allows more UV radiation to pass through the atmosphere, and (6) more UV radiation causes more skin cancer. Some ODS, including CFCs, hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform release Cl atoms. Other ODS, including halons and methyl bromide, release bromine (Br) atoms. It is these atoms that actually destroy ozone, not the intact ODS molecule. It is estimated that one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Credit: NASA GSFC.
Ozone (O3) is constantly produced and destroyed in a natural cycle called the Chapman Cycle, as shown in figure \(\PageIndex{4}\). Ozone is produced in the stratosphere by natural photochemical reactions. They involve the absorption of solar UV radiation by oxygen molecules (O2), which creates highly reactive oxygen atoms (O) that join with other O2 molecules to form O3 (figure \(\PageIndex{4}\)). Ozone (O3) is then destroyed as it absorbs further UV radiation to produce an O2 oxygen molecule and a oxygen atom (O). These reactions proceed relatively quickly in the stratosphere because high-energy UV radiation is abundant there. As a result, O3 concentrations are typically 200-300 ppb in the stratosphere, about 10 times greater than in the ambient troposphere.
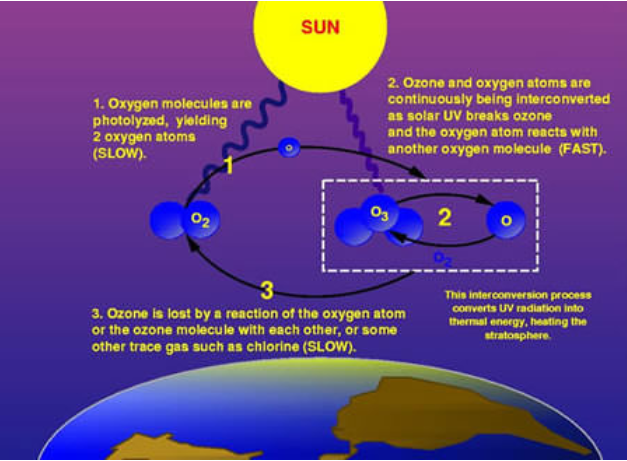
Figure \(\PageIndex{4}\): Because ozone (O3) filters out a harmful type of ultraviolet radiation (UVB), less ozone means higher UVB levels at the surface. The more the depletion, the larger the increase in incoming UVB. UVB has been linked to skin cancer, cataracts, damage to materials like plastics, and harm to certain crops and marine organisms. Although some UVB reaches the surface even without ozone depletion, its harmful effects will increase as a result of this problem. The process of the ozone layer filtering UV radiation is as follows: (1) Gaseous oxygen molecules (O2) are photolyzed (split), yielding two oxygen atoms. This is a slow process. (2) Ozone and oxygen atoms are continuously being interconverted as solar UV breaks O3 into O2 and a single oxygen atom (O). The oxygen atom (O) reacts with another O2 molecule to form O3. This interconversion is a fast process and converts UV radiation into thermal energy, heating the stratosphere. (3) Ozone is lost by a reaction of the oxygen atom or the ozone molecule with each other, or some other trace gas such as chlorine. This is a slow process.
While ozone production and destruction are balanced, ozone levels remain stable. Large increases in stratospheric ODS, however, have upset that balance. In effect, they are removing ozone faster than natural ozone creation reactions can keep up. Therefore, ozone levels fall. Stratospheric O3 can be destroyed by various processes, including reactions with the trace gases NOxand N2O and with reactive ions of bromine, chlorine, and fluorine. Because of anthropogenic emissions, the concentrations of some of these O3-consuming chemicals have been increasing in the stratosphere, leading to concerns about the depletion of stratospheric O3. It is widely believed that emissions of chlorofluorocarbons (CFCs), particularly the industrial gases known as freons, have been especially important in this regard. Because CFCs are extremely unreactive in the troposphere, they eventually migrate up to the stratosphere, where they are bombarded with UV radiation and slowly degrade (photodissociate) to release free chlorine. The chlorine efficiently reacts with and destroys O3.
The O3-destroying reactions proceed most effectively under extremely cold and stagnant conditions in the stratosphere, such as those occurring above polar latitudes at the end of the Antarctic and Arctic winters. These polar-focused O3 depletions result in the development of so-called ozone holes during the early springtime. These phenomena have been observed regularly since the early 1980s. The O3 holes over Antarctica are particularly extensive and typically involve decreases of the O3 concentration of 30-50% during the spring. Smaller depletions of O3 occur above the Arctic, including northern Canada. The affected areas in the Northern Hemisphere are much smaller than their counterparts in Antarctica. Although the seasonal losses of O3 only take place over polar regions, lower latitudes are also affected when the O3-depleted air becomes dispersed during the breakup of the holes. This temporarily reduces the stratospheric O3 concentrations throughout the hemisphere, though not nearly to the same degree as occurs in the holes themselves.
Policies to Reduce Ozone Destruction
One success story in reducing pollutants that harm the atmosphere concerns ozone-destroying chemicals. In 1973, scientists calculated that CFCs could reach the stratosphere and break apart. This would release chlorine atoms, which would then destroy ozone. Based only on their calculations, the United States and most Scandinavian countries banned CFCs in spray cans in 1978. More confirmation that CFCs break down ozone was needed before more was done to reduce production of ozone-destroying chemicals. In 1985, members of the British Antarctic Survey reported that a 50% reduction in the ozone layer had been found over Antarctica in the previous three springs.
Two years after the British Antarctic Survey report, the “Montreal Protocol on Substances that Deplete the Ozone Layer” was ratified by nations all over the world. The Montreal Protocol controls the production and consumption of 96 chemicals that damage the ozone layer. CFCs have been mostly phased out since 1995, although they were used in developing nations until 2010. Some of the less hazardous substances will not be phased out until 2030. The Protocol also requires that wealthier nations donate money to develop technologies that will replace these chemicals. Because CFCs take many years to reach the stratosphere and can survive there a long time before they break down, the ozone hole did not immediately disappear after CFC emissions were reduced; however, it has been shrinking (figure \(\PageIndex{2}\)). The ozone layer will reach the same levels it had before 1980 around 2068 and 1950 levels in one or two centuries.
CFCs have largely been replaced by hydrofluorocarbons (HFCs) in air conditioners and refrigerators. HFCs do not destroy ozone but are potent greenhouse gases.
Interactive Element
The ozone hole is shrinking due to reductions in CFC emissions. You can read more here.
Health and Environmental Effects of Ozone Layer Depletion
There are three types of UV light: UVA, UVB, and UVC. Reductions in stratospheric ozone levels will lead to higher levels of UVB reaching the Earth's surface. The sun's output of UVB does not change; rather, less ozone means less protection, and hence more UVB reaches the Earth. Studies have shown that in the Antarctic, the amount of UVB measured at the surface can double during the annual ozone hole.
Laboratory and epidemiological studies demonstrate that UVB causes non-melanoma skin cancer and plays a major role in malignant melanoma development. In addition, UVB has been linked to cataracts -- a clouding of the eye’s lens. All sunlight contains some UVB, even with normal stratospheric ozone levels. It is always important to protect your skin and eyes from the sun (see this website from the EPA on sun safety for more information). Ozone layer depletion increases the amount of UVB and the risk of health effects.
UVB is generally harmful to cells, and therefore all organisms. UVB cannot penetrate into an organism very far and thus tends to only impact skin cells. Microbes like bacteria, however, are composed of only one cell and can therefore be harmed by UVB. Physiological and developmental processes of plants are affected by UVB radiation, even by the amount of UVB in present-day sunlight. Despite mechanisms to reduce or repair these effects and a limited ability to adapt to increased levels of UVB, plant growth can be directly affected by UVB radiation. Phytoplankton form the foundation of aquatic food webs. Phytoplankton productivity is limited to the euphotic zone, the upper layer of the water column in which there is sufficient sunlight to support net productivity. The position of the organisms in the euphotic zone is influenced by the action of wind and waves. In addition, many phytoplankton are capable of active movements that enhance their productivity and, therefore, their survival. Exposure to solar UVB radiation has been shown to affect both orientation mechanisms and motility in phytoplankton, resulting in reduced survival rates for these organisms.
Contributors and Attributions
Modified by Kyle Whittinghill and Melissa Ha from the following sources:
- Ozone Depletion from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0
- Ozone in Biology by John W. Kimball, is licensed CC BY
- Gaseous Air Pollution in Environmental Science: A Canadian Perspective by Bill Freedman


