8.5: Acid Deposition
- Page ID
- 69451
Acid deposition is a term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere containing higher than normal amounts of nitric and sulfuric acids. The precursors, or chemical forerunners, of acid deposition formation result from both natural sources, such as volcanoes and decaying vegetation, and man-made sources, primarily emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) resulting from fossil fuel combustion (figure \(\PageIndex{1}\)). Acid deposition occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds. The result is a mild solution of sulfuric acid and nitric acid. When sulfur dioxide and nitrogen oxides are released from power plants and other sources, prevailing winds blow these compounds across state and national borders, sometimes over hundreds of miles.
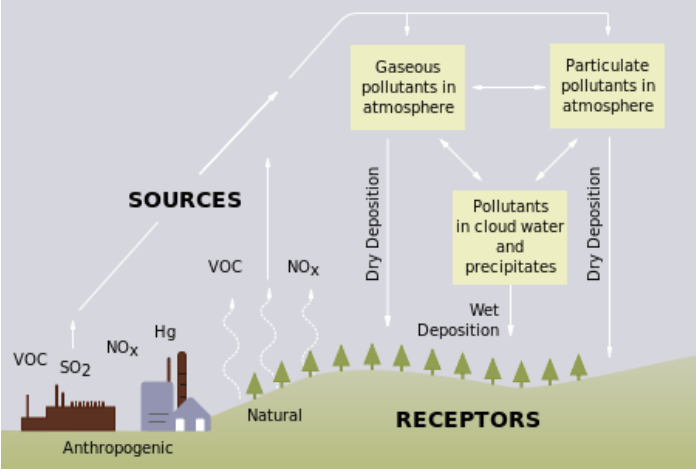
Figure \(\PageIndex{1}\): This diagram shows both anthropogenic and natural sources of acid deposition. Volatile organic compounds (VOCs), sulfur dioxide (SO2), nitrogen oxides (NOX), and mercury (Hg) are released, but only SO2 and NOX react to form acids. Three categories of pollutants interact with each other. These are gaseous pollutants in the atmosphere, particulate pollutants in the atmosphere, and pollutants in cloud water and precipitates. The former two categories form dry deposition, and the latter forms wet deposition. Acid deposition (both dry and wet deposition) reaches natural areas, which could be terrestrial (as pictured) or aquatic.
Acid deposition is measured using a scale called “pH.” The lower a substance’s pH, the more acidic it is. Pure water has a pH of 7.0. However, normal rain is slightly acidic because carbon dioxide (CO2) dissolves into it forming weak carbonic acid, giving the resulting mixture a pH of approximately 5.6 at typical atmospheric concentrations of CO2. The average pH of precipitation in the United States in 1980 was 4.6, but it has been gradually increasing due to reductions in sulfur dioxide and nitrogen oxide emissions (figure \(\PageIndex{2}\)).
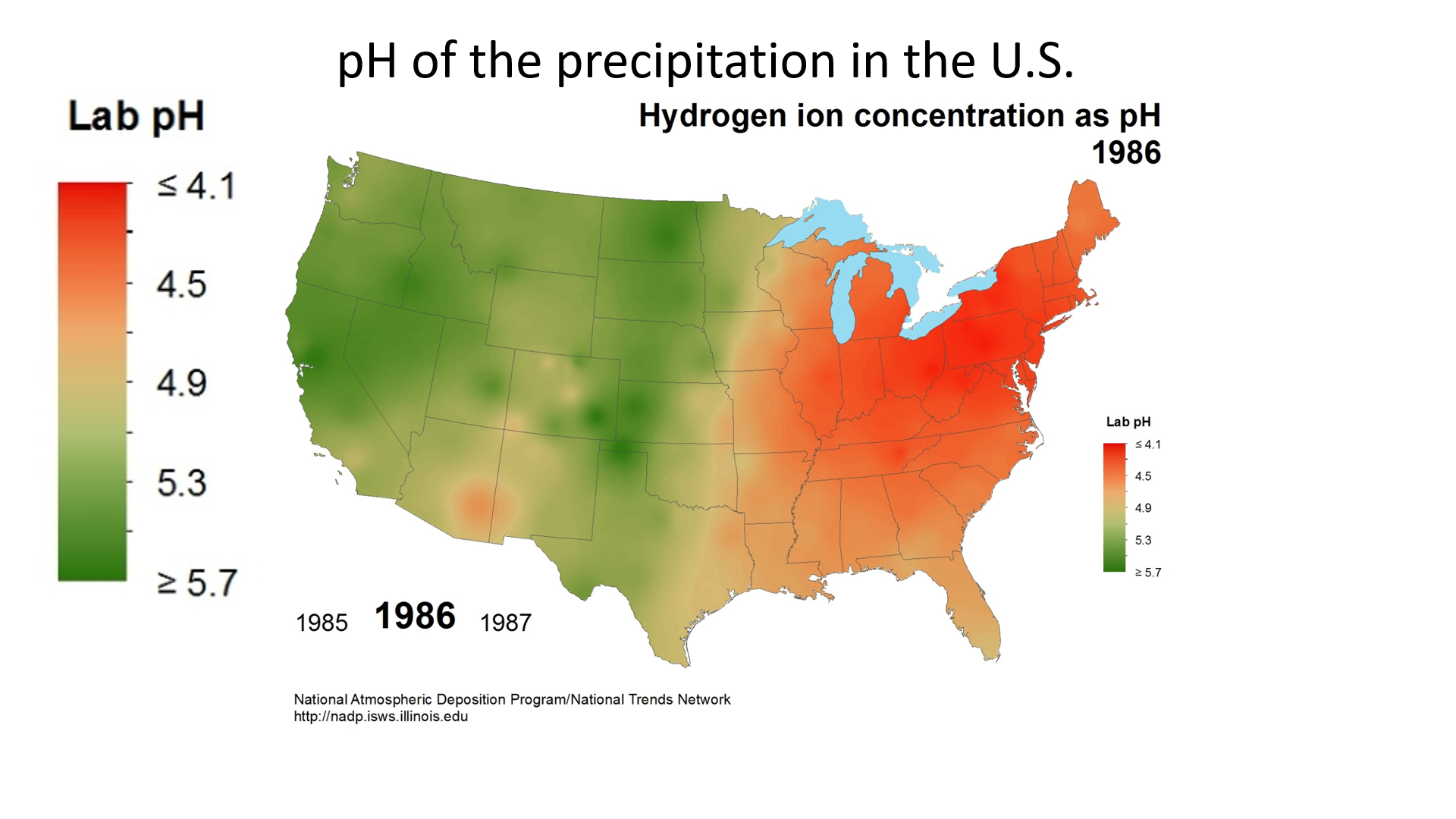
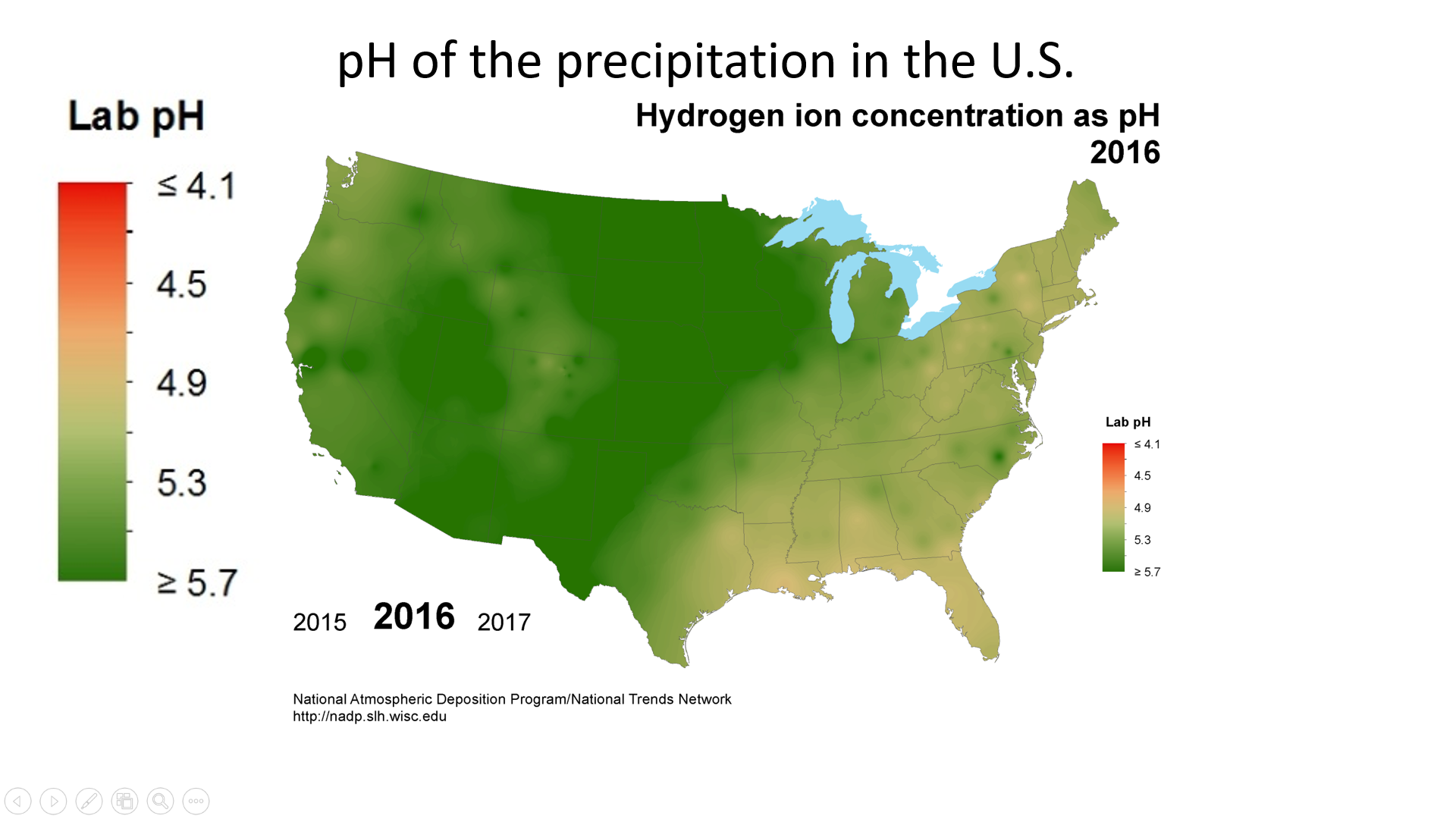
Figure \(\PageIndex{2}\): pH measures how acidic or basic a solution is, which depends on the concentration of hydrogen ions. Precipitation is normally slightly acidic, with a pH of around 5.6. (Anything below pH = 7 is considered acidic.) pH of precipitation was even more acidic in much of the East Coast of the U.S. in 1986 (acid deposition) with parts of Michigan, Ohio, Pennsylvania, and New York state reaching a pH of less than or equal to 4.1. The pH of the western United States in 1986 was closer to 5.0. Due to efforts to reduce air pollution, the pH of precipitation has moved closer to normal. By 2016, the pH of the eastern United States had increased to around 4.9, and the pH of the western United States had mostly returned to normal, with many regions having a pH of greater than or equal to 5.7. Images by National Atmospheric Deposition Program (public domain).
Effects of Acid Deposition
Acid deposition causes acidification of lakes and streams and contributes to the damage of trees at high elevations (for example, red spruce trees above 2,000 feet) and many sensitive forest soils. In addition, acid deposition accelerates the decay of building materials and paints, including irreplaceable buildings, statues, and sculptures that are part of our nation’s cultural heritage. Prior to falling to the earth, sulfur dioxide (SO2) and nitrogen oxide (NOx) gases and their particulate matter derivatives—sulfates and nitrates—contribute to visibility degradation and harm public health.
The ecological effects of acid deposition are most clearly seen in the aquatic, or water, environments, such as streams, lakes, and marshes. Most lakes and streams have a pH between 6 and 8, although some lakes are naturally acidic even without the effects of acid deposition. Acid deposition primarily affects sensitive bodies of water, which are located in watersheds whose soils have a limited ability to neutralize acidic compounds (called “buffering capacity”). Lakes and streams become acidic (i.e., the pH value goes down) when the water itself and its surrounding soil cannot buffer the acid deposition enough to neutralize it. In areas where buffering capacity is low, acid deposition releases aluminum from soils into lakes and streams; aluminum is highly toxic to many species of aquatic organisms. Acid deposition causes slower growth, injury, or death of forests. Of course, acid deposition is not the only cause of such conditions. Other factors contribute to the overall stress of these areas, including air pollutants, insects, disease, drought, or very cold weather. In most cases, in fact, the impacts of acid deposition on trees are due to the combined effects of acid deposition and these other environmental stressors.
Acid deposition contributes to the corrosion of metals (such as bronze) and the deterioration of paint and stone (such as marble and limestone). These effects significantly reduce the societal value of buildings, bridges, cultural objects (such as statues, monuments, and tombstones), and cars (figure \(\PageIndex{3}\)).

Figure \(\PageIndex{3}\): A gargoyle that has been damaged by acid deposition.
Sulfates and nitrates that form in the atmosphere from sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions contribute to visibility impairment, meaning we cannot see as far or as clearly through the air. The pollutants that cause acid deposition—sulfur dioxide (SO2) and nitrogen oxides (NOx)—damage human health. These gases interact in the atmosphere to form fine sulfate and nitrate particles that can be transported long distances by winds and inhaled deep into people’s lungs. Fine particles can also penetrate indoors. Many scientific studies have identified a relationship between elevated levels of fine particles and increased illness and premature death from heart and lung disorders, such as asthma and bronchitis.
Contributors and Attribution
Modified by Kyle Whittinghill and Melissa Ha from the following sources
- Acid Rain from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0.


