5.8: Sulfur Cycle
- Page ID
- 76515
Key Points
- Sulfur is an essential element for the macromolecules of living things since it determines the 3-D folding patterns of proteins.
- On land, sulfur enters the atmosphere via acid rain, fallout, the weathering of rocks, decompostion of organic materials, and geothermal vents.
- Sulfur enters the ocean via runoff, fallout, and underwater geothermal vents; some marine ecosystems also rely on chemoautotrophs as a sulfur source.
- The burning of fossil fuels increases the amount of sulfide in the atmosphere and causes acid rain.
- Acid rain is corrosive rain that causes damage to aquatic ecosystems by lowering the pH of lakes, killing many of the resident fauna; it also degrades buildings and human-made structures.
The Sulfur Cycle
Sulfur is an essential element for the macromolecules of living things. As a part of the amino acid cysteine, it is involved in the formation of disulfide bonds within proteins, which help to determine their 3-D folding patterns and, hence, their functions. As shown in Figure \(\PageIndex{1}\), sulfur cycles between the oceans, land, and atmosphere. Atmospheric sulfur is found in the form of sulfur dioxide (SO2), which enters the atmosphere in three ways: first, from the decomposition of organic molecules; second, from volcanic activity and geothermal vents; and, third, from the burning of fossil fuels by humans.
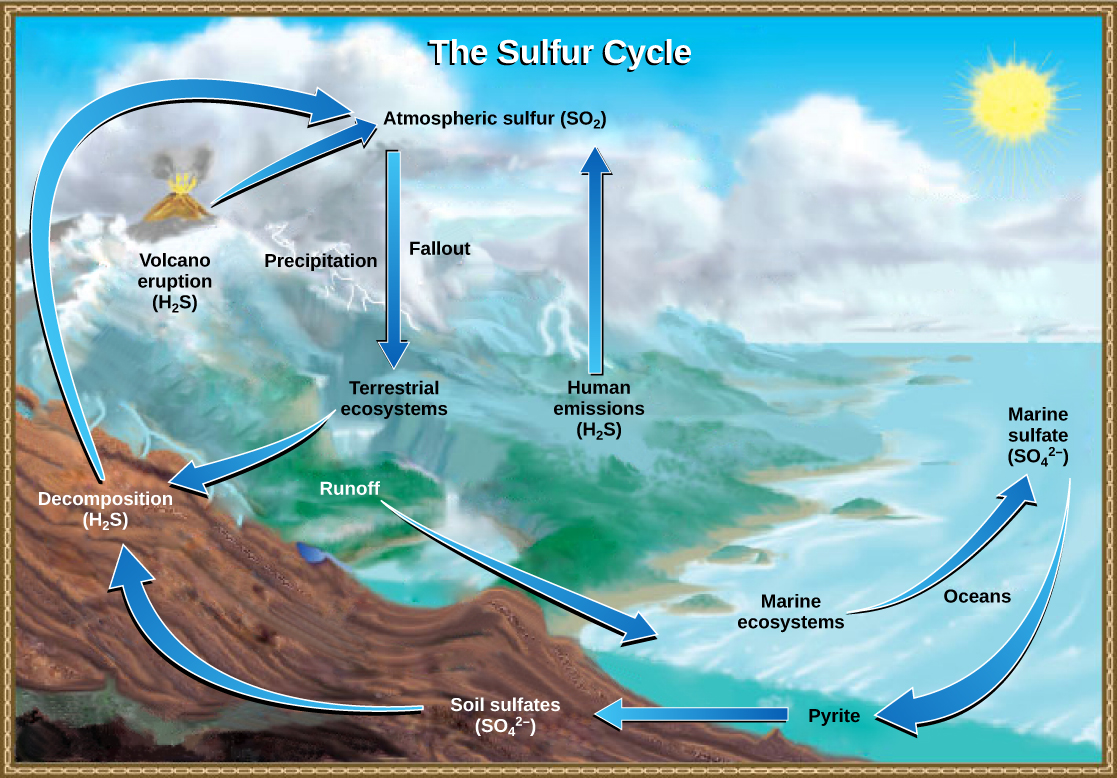
On land, sulfur is deposited in five major ways: precipitation, direct fallout from the atmosphere, rock weathering, decomposition of organic material and geothermal vents (Figure \(\PageIndex{2}\)). As rain falls through the atmosphere, sulfur dioxide (SO2) in the atmosphere is dissolved in the form of weak sulfuric acid (H2SO4), creating acid rain. Sulfur can also fall directly from the atmosphere in a process called fallout. The weathering of sulfur-containing rocks also releases sulfur into the soil. These rocks originate from ocean sediments that are moved to land by the geologic uplift. Terrestrial ecosystems can then make use of these soil sulfates (SO42-), which enter the food web by being taken up by plant roots. When these plants decompose and die, sulfur is released back into the atmosphere as hydrogen sulfide (H2S) gas.

Sulfur enters the ocean in runoff from land, from atmospheric fallout, and from hydrothermal vents. Some ecosystems rely on microorganisms using sulfur as a biological energy source (chemoautotrophs) in contrast to ecosystems with photosynthetic producers. This sulfur then supports marine ecosystems in the form of sulfates.
Human activities have played a major role in altering the balance of the global sulfur cycle. The burning of large quantities of fossil fuels, especially from coal, releases sulfur dioxide, which reacts with the atmosphere to form sulfuric acid. Like nitric acid, sulfuric acid contributes to acid deposition. Acid deposition (sometimes referred to simply as acid rain) damages the natural environment by lowering the pH of lakes, thus killing many of the resident plants and animals. Acid deposition also affects the man-made environment through the chemical degradation of buildings. For example, many marble monuments, such as the Lincoln Memorial in Washington, DC, have suffered significant damage from acid rain over the years. These examples show the wide-ranging effects of human activities on our environment and the challenges that remain for our future.
Chemosynthesis
Why do bacteria that live deep below the ocean’s surface rely on chemical compounds instead of sunlight for energy to make food?
Most autotrophs make food by photosynthesis, but this isn’t the only way that autotrophs produce food. Some bacteria make food by another process, which uses chemical energy instead of light energy. This process is called chemosynthesis. In chemosynthesis, one or more carbon molecules (usually carbon dioxide or methane, CH4) and nutrients is converted into organic matter, using the oxidation of inorganic molecules (such as hydrogen gas, hydrogen sulfide (H2S) or ammonia (NH3)) or methane as a source of energy, rather than sunlight. In hydrogen sulfide chemosynthesis, in the presence of carbon dioxide and oxygen,carbohydrates (CH2O) can be produced:
CO2 + O2 + 4H2S → CH2O + 4S + 3H2O
Many organisms that use chemosynthesis are extremophiles, living in harsh conditions, such as in the absence of sunlight and a wide range of water temperatures, some approaching the boiling point. Some chemosynthetic bacteria live around deep-ocean vents known as “black smokers.” Compounds such as hydrogen sulfide, which flow out of the vents from Earth’s interior, are used by the bacteria for energy to make food. These organisms are known as chemoautotrophs. Many chemosynthetic microorganisms are consumed by other organisms in the ocean, and symbiotic associations between these organisms and respiring heterotrophs are quite common. Consumers that depend on these bacteria to produce food for them include giant tubeworms (Figure \(\PageIndex{3}\)), and Pompeii worms ((Figure \(\PageIndex{4}\)).
Figure \(\PageIndex{3}\): Tubeworms deep in the Galapagos Rift get their energy from chemosynthetic bacteria. Tubeworms have no mouth, eyes or stomach. Their survival depends on a symbiotic relationship with the billions of bacteria that live inside them. These bacteria convert the chemicals that shoot out of the hydrothermal vents into food for the worm.
Figure \(\PageIndex{3}\): Is it possible to live in temperatures over 175°F? It is if you're a Pompeii worm. The Pompeii worm, the most heat-tolerant animal on Earth, lives in the deep ocean at super-heated hydrothermal vents. Covering this deep-sea worm's back is a fleece of bacteria. These microbes contain all the genes necessary for life in extreme environments.
Contributors and Attributions
This section was modified from the original by Kyle Whittinghill
Samantha Fowler (Clayton State University), Rebecca Roush (Sandhills Community College), James Wise (Hampton University). Original content by OpenStax (CC BY 4.0; Access for free at https://cnx.org/contents/b3c1e1d2-83...4-e119a8aafbdd).
- 20.2: Biogeochemical Cycles by OpenStax, is licensed CC BY
- 2.24: Chemosynthesis by CK-12: Biology Concepts, is licensed CC BY-NC
- General Microbiology Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
- Atmosphere-Biosphere-Hydrosphere-Lithosphere. Provided by: Wikimedia. Located at: commons.wikimedia.org/wiki/Fi...ithosphere.png. License: CC BY: Attribution

