Introduction to Electron Transport Chains and Respiration*#
- Page ID
- 14529
Section Overview
In the next few modules, we start to learn about the process of respiration and the roles that electron transport chains play in this process. A definition of the word "respiration" that most people are familiar with is "the act of breathing". When we breath, air including molecular oxygen is brought into our lungs from outside of the body, the oxygen then becomes reduced, and waste products, including the reduced oxygen in the form of water, are exhaled. More generically, some reactant comes into the organism and then gets reduced and leaves the body as a waste product.
This generic idea, in a nutshell, can be generally applied across biology and oxygen need not always be the compound that brought in, reduced, and dumped as waste. The electrons that are dumped on oxygen or other compounds more generally known as "terminal electron acceptors." The molecules from which the electrons that are dumped onto terminal electron acceptors originate, vary greatly across biology (we have looked at one possible source - the reduced carbon-based molecule glucose).
In between the original electron source and the terminal electron acceptor are a series of biochemical reactions involving at least one redox reaction. These redox reactions harvest energy for the cell by coupling exergonic redox reaction to an energy-requiring reaction in the cell. In respiration, a special set of enzymes carry out a linked series of redox reactions that ultimately transfer electrons to the terminal electron acceptor.
These "chains" of redox enzymes and electron carriers are called electron transport chains (ETC). ETCs are therefore the portion of respiration that use an electron acceptor (usually brought in from outside of the cell) as the final/terminal acceptor for the electrons that were removed from the intermediate compounds in catabolism. In aerobically respiring eukaryotic cells the ETC is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them. The electrons are passed from enzyme to enzyme through a series of redox reactions. These reactions are couple the exergonic redox transfers to the endergonic transport of hydrogen ions across the membrane. This process contributes to the creation of a transmembrane electrochemical gradient. The electrons passing through the ETC gradually lose potential energy up until the point they are deposited on the terminal electron acceptor which is typically removed as waste from the cell. When oxygen as the final electron acceptor, the free energy difference of this multistep redox process is ~ -60 kcal/mol when NADH donates electrons or 45 kcal/mol when FADH2 donates.
Introduction to redox, oxidative phosphorylation and Electron Transport Chains
In prior modules we discussed the general concept of redox reactions in biology and introduced the Electron Tower, a tool to help you understand redox chemistry and to estimate the direction and magnitude of potential energy differences for various redox couples. In later modules, substrate level phosphorylation and fermentation were discussed and we saw how exergonic redox reactions could be directly coupled by enzymes to the endergonic synthesis of ATP.
These processes are hypothesized to be one of the oldest forms of energy production used by cells. In this section we discuss the next evolutionary advancement in cellular energy metabolism, oxidative phosphorylation. First and foremost, oxidative phosphorylation does not imply the use of oxygen, it can, but it does not have to use oxygen. It is called oxidative phosphorylation because it relies on redox reactions to generate a electrochemical transmembrane potential that can then be used by the cell to do work.
A quick summary of Electron Transport Chains
The ETC begins with the addition of electrons, donated from NADH, FADH2 or other reduced compounds. These electrons move through a series of electron transporters, enzymes that are embedded in a membrane, or carriers that undergo redox reactions. The free energy transferred from these exergonic redox reactions is often coupled to the endergonic movement of protons across a membrane. Since the membrane is an effective barrier to charged species, this pumping results in an unequal accumulation of protons on either side of the membrane. This in turn "polarizes" or "charges" the membrane, with a net positive (protons) on one side of the membrane and a negative charge on the other side of the membrane. The separation of charge creates an electrical potential. In addition, the accumulation of protons also causes a pH gradient known as a chemical potential across the membrane. Together these two gradients (electrical and chemical) are called an electro-chemical gradient.
Review: The Electron Tower
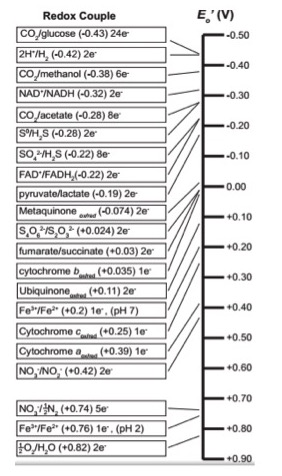
Since redox chemistry is so central to the topic we begin with a quick review of the table of reduction potential - sometimes called the "redox tower" or "electron tower". You may hear your instructors use these terms interchangeably. As we discussed in previous modules, all kinds of compounds can participate in biological redox reactions. Making sense of all of this information and ranking potential redox pairs can be confusing. A tool has been developed to rate redox half reactions based on their reduction potentials or E0' values. Whether a particular compound can act as an electron donor (reductant) or electron acceptor (oxidant) depends on what other compound it is interacting with. The redox tower ranks a variety of common compounds (their half reactions) from most negative E0', compounds that readily get rid of electrons, to the most positive E0', compounds most likely to accept electrons. The tower organizes these half reactions based on the ability of electrons to accept electrons. In addition, in many redox towers each half reaction is written by convention with the oxidized form on the left followed by the reduced form to its right. The two forms may be either separated by a slash, for example the half reaction for the reduction of NAD+ to NADH is written: NAD+/NADH + 2e-, or by separate columns. An electron tower is shown below.
Note
Use the redox tower above as a reference guide to orient you as to the reduction potential of the various compounds in the ETC. redox reactions may be either exergonic or endergonic depending on the relative redox potentials of the donor and acceptor. Also remember there are many different ways of looking at this conceptually; this type of redox tower is just one way.
Note: Language shortcuts reappear
In the redox table above some entries seem to be written in unconventional ways. For instance Cytochrome cox/red. There only appears to be one form listed. Why? This is another example of language shortcuts (likely because someone was too lazy to write cytochrome twice) that can be confusing - particularly to students. The notation above could be rewritten as Cytochrome cox/Cytochrome cred to indicate that the cytochrome c protein can exist in either and oxidized state Cytochrome cox or reduced state Cytochrome cred.
Review Redox Tower Video
For a short video on how to use the redox tower in redox problems click here. This video was made by Dr. Easlon for Bis2A students.
Using the redox tower: A tool to help understand electron transport chains
By convention the tower half reactions are written with the oxidized form of the compound on the left and the reduced form on the right. Notice that compounds such as glucose and hydrogen gas are excellent electron donors and have very low reduction potentials E0'. Compounds, such as oxygen and nitrite, whose half reactions have relatively high positive reduction potentials (E0') generally make good electron acceptors are found at the opposite end of the table.
Example: Menaquinone
Let's look at menaquinoneox/red. This compound sits in the middle of the redox tower with an half-reaction E0' value of -0.074 eV. Menaquinoneox can spontaneously (ΔG<0) accept electrons from reduced forms of compounds with lower half-reaction E0'. Such transfers form menaquinonered and the oxidized form of the original electron donor. In the table above, examples of compounds that could act as electron donors to menaquinone include FADH2, an E0' value of -0.22, or NADH, with an E0' value of -0.32 eV. Remember the reduced forms are on the right hand side of the red/ox pair.
Once menaquinone has been reduced, it can now spontaneously (ΔG<0) donate electrons to any compound with a higher half-reaction E0' value. Possible electron acceptors include cytochrome box with an E0' value of 0.035 eV; or ubiquinoneox with an E0' of 0.11 eV. Remember that the oxidized forms lie on the left side of the half reaction.