pH_Mary_Caidon
- Page ID
- 39850
The Role of Acid/Base Chemistry in General Biology
We have learned that the behavior of chemical functional groups depends on the composition, order, and properties of their constituent atoms. We will see that pH, a measure of the hydrogen ion concentration of a solution, can alter the chemical properties of some key biological functional groups in ways that change how they interact with other molecules and thus their biological role.
For example, depending on the pH, some functional groups on the amino acid that make up proteins can exist in different chemical states. We will learn that the chemical state of these functional groups can have a profound effect on the shape of the protein or on its ability to carry out chemical reactions. As we move through the course, we will see many examples of this type of chemistry in different contexts.
In pure water, hydrogen ions are spontaneously generated by the dissociation (ionization) of a small percentage of water molecules into equal numbers of hydrogen (H+) ions and hydroxide (OH-) ions. The OH- that result from the ionization of water departs into the sea of water molecules interacting with other molecules through polar interactions, while the now "free" (unbonded) H+ ions produced by the ionization associates with water molecules (line two of the figure below) to create a new molecule called a hydronium ion, H3O+. At some point, the hydroxide ion from line 1 in the figure below will rejoin with a proton and reform another water molecule. This process of dissociation and re-association between hydroxide and hydrogen ions happens continuously at equilibrium.
While most H+ ions in solution really exist as H3O+ ions, we usually represent the H3O+ in figures or equations more simply as H+. Why? Because it is easier. Just remember that since nearly all chemistry in biology happens in water that when you see H+ referred to in the text, figures, or in equations, it usually represents H3O+.

Figure 1: Water spontaneously dissociates into a proton and hydroxyl group. The proton will combine with a water molecule forming a hydronium ion.
Attribution: Marc T. Facciotti
While some paradoxes to this rule can be found in the chemistry of concentrated solutions, in General Biology, it is convenient to formally define pH as:
|
\[ pH = -\log_{10} [H^+]\]
|
In the equation above, the square brackets surrounding [H+] indicate concentration. If necessary, try a math review at wiki-logarithm or kahn-logarithm. Also see: definition-concentration or wiki-concentration. The pH of a solution is therefore a measure of the concentration of hydrogen ions in a solution (or the number of hydronium ions).
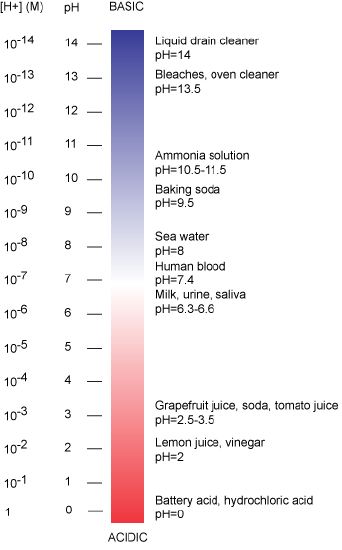
The pH is typically reported on a logarithmic pH scale that ranges from 0 to 14 (Figure 2). We define pH=7.0 as neutral. We call anything with a pH below 7.0 acidic and any reported pH above 7.0 alkaline or basic. Extremes in pH in either direction from 7.0 are often considered inhospitable to life, although examples exist to the contrary. pH levels in the human body usually range between 6.8 and 7.4, except in the stomach where the pH is more acidic, typically between 1 and 2. Some microbial species like Sulfolobus acidocaldarius thrive in hyper acidic environments (pH < 3) while others like Natronomonas pharaonis have been found living in lakes with pH > 11. These organisms are classified as "extremophiles" for their abilities to thrive in extreme environments. Proteins from these organisms are sometimes used in industrial processes where their ability to withstand environmental stress is a valued property.
Figure 2: The pH scale ranging from acidic to basic with various biological compounds or substances that exist at that particular pH. Attribution: Marc T. Facciotti
For Additional Information
Watch this video for an expanded explanation of pH and its relationship to [H+] and the logarithmic scale.
Let's work out an example to see how the pH scale works.
For reference: 1 mole (mol) of a substance (which can be atoms, molecules, ions, etc.), is defined as being equal to 6.02 x 1023 particles of the substance. Therefore, 1 mole of water is equal to 6.02 x 1023 water molecules.
Mathematically this can be written as:
1 mol = 6.02x1023 particles in a substance
1 mol H2O = 6.02x1023 water molecules
Example: The concentration of hydrogen ions dissociating from pure water is approximately 1 × 10-7 moles H+ ions per liter of water. The pH is calculated as the negative of the base 10 logarithm of this unit of concentration. The log10 of 1×10-7 is -7.0, and the negative of this number yields a pH of 7.0 (neutral pH).
Mathematically this can be represented as:
pH = -log10[H+]
pH = -log10[1×10-7]
pH = 7.0 (neutral pH)
The figure below provides another way to visualize the inverse relationship between proton and hydroxide ion concentrations by graphically illustrating how proton concentration decreases as pH increases while the hydroxide ion concentration simultaneously increases.
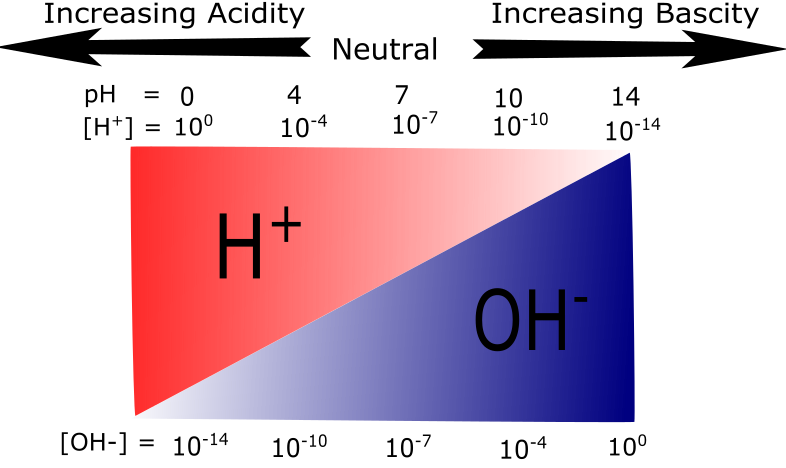
Figure 3: A graphical representation of acidity and basicity. This figure illustrates the relationship between H+ and OH- concentrations on the pH scale. At low pH values H+ ions are plentiful. As the pH increases the relative abundance of OH- ions increase while H+ abundance decreases.
Attribution: Mary O. Aina
The inverse relationship between pH and the concentration of protons confuses many students - take the time to convince yourself that you "get it." One way could be to predict whether different pH values are acidic or basic and then do the calculations to make sure. Start by trying these practice questions.
Knowledge Check Quiz
Acids and Bases
Acids and bases are molecules that can influence the pH of a solution. In General Biology it is often convenient to use the Brønsted-Lowry definition of acids and bases. Using this formalism we define:
Acids = molecules that can donate a proton to another molecule (including water to form a hydronium ion)
Bases = molecules that can accept a proton from another molecule (including hydronium ions)
When protons from acidic molecules dissociate from their "parent" they increase the H+ concentration and thereby lower the pH of the solution. By contrast, when a base absorbs a "free" proton from a solution onto the "parent" molecule, the decrease in proton concentration in solution results in a shift to higher pH values.
Generically we can represent acids and bases as follows:
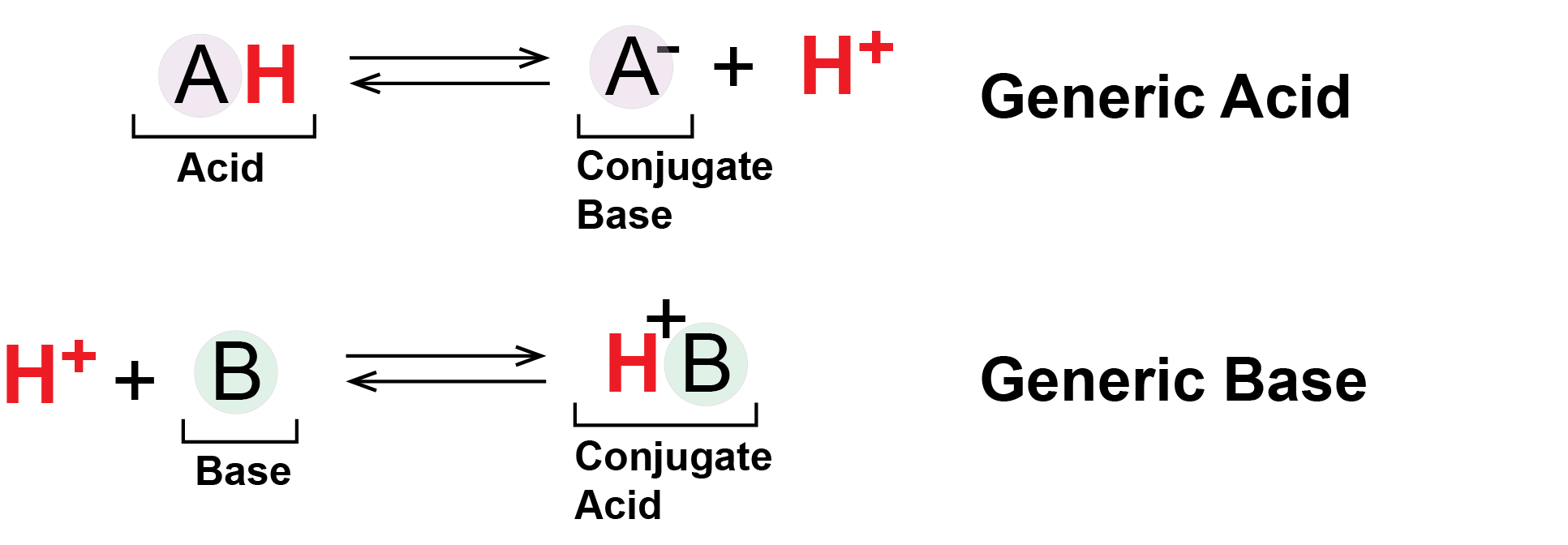
Figure 4: Generic Acids and Bases. This figure shows the behavior of Brønsted-Lowry acids and bases. The acid (A in a light purple circle) starts in a protonated form bound to an H+ ion, drawn as a red H. The acid deprotonates, shedding its H+ into solution or to another molecule. Meanwhile the base (B in a light green circle) begins deprotonated and absorbs a proton (red H+) from solution or other molecule.
Attribution: Marc T. Facciotti
In the figure above, the molecule A- - the deprotonated form of the acid AH - can also be referred to as the conjugate base of the acid AH. Likewise the molecule BH+ - the protonated form of the base B - can be referred to as the conjugate acid of the base B.
Two important examples of weak acids/bases in biology are the carboxyl and amino functional groups. At physiological pH values (around pH = 7) the carboxyl group tends to behave as an acid by donating it's proton to solution or other molecules. Under the same conditions, the amino group tends to act as a base, absorbing protons from solution or other molecules. As we will soon see, these and other protonation/deprotonation reactions play key roles in many biological processes.
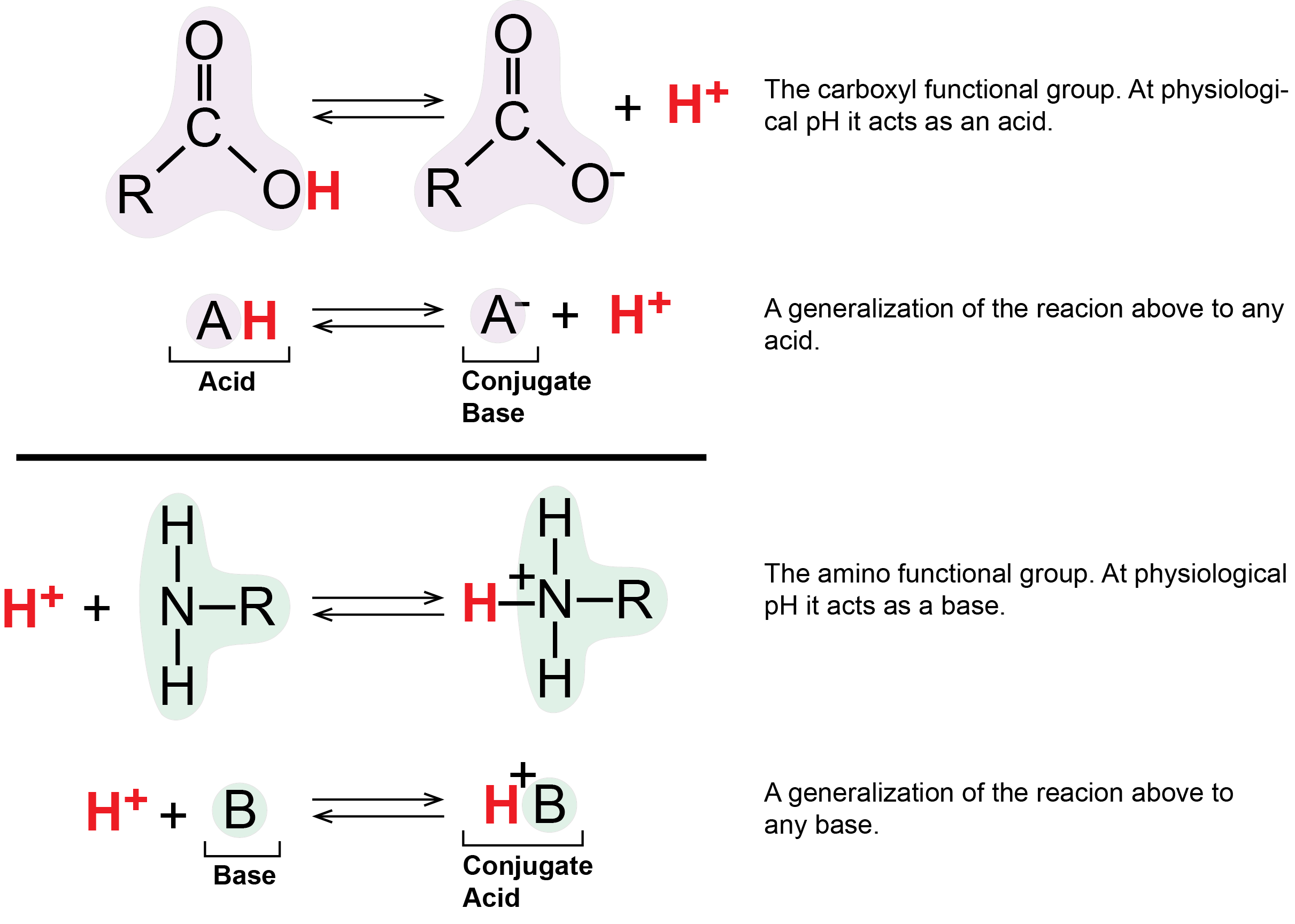
Figure 5: The carboxylic acid group acts as an acid by releasing a proton. This can increase the number of protons in solution and thus decrease the pH. The amino group acts as a base by accepting hydrogen ions, which can decrease the number of hydrogen ions in solutions, thus increasing the pH.
Attribution: Marc T. Facciotti (original work)
Additional pH resources
Here are some additional links on pH and pKa to help learn the material. Note that there is an additional module devoted to pKa.
ChemLibreText Links
- Determining and calculating pH
- pH and pKa
- This has an expanded discussion about pH that is a bit more detailed than how we present the concept.
Khan Academy Links
Simulations
- Acid-base simulation. -------
- ----------embed this in document---------