Electron Carriers*#
- Page ID
- 21277
Red/ox chemistry and electron carriers
The oxidation of, or removal of an electron from, a molecule (whether accompanied with the removal of an accompanying proton or not) results in a change of free energy for that molecule—matter, internal energy, and entropy have all changed. Likewise, the reduction of a molecule also changes its free energy. The magnitude of change in free energy and its direction (positive or negative) for a red/ox reaction dictates the spontaneity of the reaction and how much energy it transfers. In biological systems, where a great deal of energy transfer happens via red/ox reactions, it is important to understand how these reactions
Note: BURNING BEAR
Relate the burning of (the full oxidation of the sugar in) a gummy bear with the last paragraph above. What does that demonstration have to do with our upcoming discussion on red/ox carriers? There is some mention above already—can you find it?
Note: DESIGN CHALLENGE
The problem alluded to in the previous discussion question is a great place to bring in the design challenge rubric. If you recall, the first step of the rubric asks that you define a problem or question. Here, let's imagine that there is a problem to define for which the mobile electron carriers below helped Nature solve.
***Remember, evolution DOES NOT forward-engineer solutions to problems, but in retrospect, we can use our imagination and logic to infer that what we see preserved by natural selection provided a selective advantage, because the natural innovation "solved" a problem that limited success.***
Design challenge for red/ox carriers
- What was a problem
( s) that the evolution of mobile electron carriers helped solve? - The next step of the design challenge asks you to identify criteria for successful solutions. What are criteria for success in the problem you've identified?
- Step 3 in the design challenge asks you to identify solutions. Well, here Nature has identified some for us—we consider three in the reading below. It looks like Nature is happy to have multiple solutions to the problem.
- The penultimate step of the design challenge rubric asks you to test the proposed solutions against the criteria for success. This should make you think/discuss why there are multiple different electron carriers. Are there different criteria for success? Are they each solving slightly different problems? What do you think? Be on the lookout as we go through metabolism for clues.
NAD+/H and FADH/H2
In living systems, a small class of compounds function as electron shuttles: they bind and carry electrons between compounds in different metabolic pathways. The principal electron carriers we will consider derive from the B vitamin group and nucleotides. These compounds can both become reduced (that is, they accept electrons) or oxidized (they lose electrons) depending on the reduction potential of a potential electron donor or acceptor that they might transfer electrons to and from. Nicotinamide adenine dinucleotide (NAD+) (we show the structure below) derives from vitamin B3, niacin. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron).
We are expecting you to memorize the two forms of NAD+/NADH, know which form is oxidized and which is reduced, and be able to recognize either form on the spot in a chemical reaction.
NAD+ can accept electrons from an organic molecule according to the general equation:

Here is some vocabulary review: when electrons are added to a compound, we say the compound has been reduced. A compound that reduces (donates electrons to) another is called a reducing agent. In the above equation, RH is a reducing agent, and NAD+ becomes reduced to NADH. When electrons leave a compound, it becomes oxidized. A compound that oxidizes another is called an oxidizing agent. In the above equation, NAD+ is an oxidizing agent, and RH is oxidized to R. Put another way, the reducing agent gets oxidized and the oxidizing agent gets reduced.
You need to get this down! We will (a) test specifically on your ability to do so (as "easy" questions), and (b) we will use the terms with the expectation that you know what they mean and can relate them to biochemical reactions correctly (in class and on tests).
You will also encounter a second variation of NAD+, NADP+. It is structurally very similar to NAD+, but it contains an extra phosphate group and plays an important role in anabolic reactions, such as photosynthesis. Another nucleotide-based electron carrier that you will also encounter in this course and beyond, flavin adenine dinucleotide (FAD+), derives from vitamin B2, also called riboflavin. Its reduced form is FADH2. Learn to recognize these molecules as electron carriers.

Figure 1. The oxidized form of the electron carrier (NAD+) is shown on the left, and the reduced form (NADH) is shown on the right. The nitrogenous base in NADH has one more hydrogen ion and two more electrons than in NAD+.
The cell uses NAD+ to "pull" electrons off of compounds and to "carry" them to other locations within the cell; thus we call it an electron carrier. Many metabolic processes we will discuss in this class involve NAD(P)+/H compounds. For example, in its oxidized form, NAD+ is used as a reactant in glycolysis and the TCA cycle, whereas in its reduced form (NADH), it is a reactant in fermentation reactions and electron transport chains (ETC). We will discuss each of these processes in later modules.
Energy story for a red/ox reaction
***As a rule of thumb, when we see NAD+/H as a reactant or product, we know we are looking at a red/ox reaction.***
When NADH is a product and NAD+ is a reactant, we know that NAD+ has become reduced (forming NADH); therefore, the other reactant must have been the electron donor and become oxidized. The reverse is also true. If NADH has become NAD+, then the other reactant must have gained the electron from NADH and become reduced.
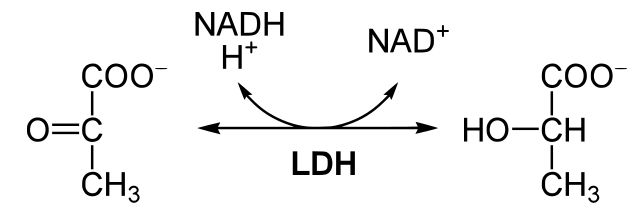
Figure 2. This reaction shows the conversion of pyruvate to lactic acid coupled with the conversion of NADH to NAD+. Source: https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/sequential_reactions
In the figure above, we see pyruvate becoming lactic acid, coupled with the conversion of NADH into NAD+. LDH catalyses this reaction. Using our "rule of thumb" above, we categorize this reaction as a red/ox reaction. NADH is the reduced form of the electron carrier, and NADH is converted into NAD+. This half of the reaction results in the oxidation of the electron carrier. Pyruvate is converted into lactic acid in this reaction. Both sugars are negatively charged, so it would be difficult to see which compound is more reduced using the charges of the compounds. However, we know that pyruvate has become reduced to form lactic acid, because this conversion is coupled to the oxidation of NADH into NAD+. But how can we tell that lactic acid is more reduced than pyruvate? The answer is to look at the carbon-hydrogen bonds in both compounds. As electrons transfer, they are often accompanied by a hydrogen atom. Pyruvate has a total of three C-H bonds, while lactic acid has four C-H bonds. When we compare these two compounds in the before and after states, we see that lactic acid has one more C-H bond; therefore, lactic acid is more reduced than pyruvate. This holds true for multiple compounds. For example, in the figure below, you should be able to rank the compounds from most to least reduced using the C-H bonds as your guide.

Figure 3. Above are a series of compounds than can be ranked or reorganized from most to least reduced. Compare the number of C-H bonds in each compound. Carbon dioxide has no C-H bonds and is the most oxidized form of carbon we will discuss in this class. Answer: the most reduced is methane (compound 3), then methanol (4), formaldehyde (1), carboxylic acid (2), and finally carbon dioxide (5).

Figure 4. This reaction shows the conversion of G3P, NAD+, and Pi into NADH and 1,3-BPG. This reaction is catalyzed by glyceraldehyde-3-phosphate dehydrogenase.
Energy story for the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase:
Lets make an energy story for the reaction above.
First, let us characterize the reactants and products. The reactants are glyceraldehyde-3-phosphate (a carbon compound), Pi (inorganic phosphate), and NAD+. These three reactants enter a chemical reaction to produce two products, NADH and 1,3-bisphosphoglycerate. If you look closely, you can see that the 1,3-BPG contains two phosphates. This is important since a chemical reaction should lose no mass between its beginning and its end. There are two phosphates in the reactants, so there must be two phosphates in the products (conservation of mass!). You can double check the book keeping of mass for all other atoms. It should also tabulate correctly. An enzyme called glyceraldehyde-3-phosphate dehydrogenasethat catalyzes this reaction. The standard free energy change of this reaction is ~6.3 kJ/mol, so under standard conditions, we can say that the free energy of the products is higher than that of the reactants and that this reaction is not spontaneous under standard conditions.
What can we say about this reaction when it is catalyzed by glyceraldehyde-3-phosphate dehydrogenase?
This is a red/ox reaction. We know that because we have produced a reduced electron carrier (NADH) as a product and NAD+ is a reactant. Where did the electron come from to make NADH? The electron must have come from the other reactant (the carbon compound).



