Alternative View of Some Common Confusing Issues in Basic Redox Chemistry for Biology
- Page ID
- 41428
Alternative View of Some Common Confusing Issues in Basic Redox Chemistry for Biology
This reading tries to break down some of the more challenging topics that students encounter when studying redox chemistry in General Biology. This reading is not a substitute for your main reading but rather a complement to it that revisits some of the same topics through a different lens.
Finding ΔE
Students often struggle with finding the ∆E for a given redox reaction. One of the main barriers to developing this skill seems to be associated with developing a picture of the redox reaction itself. From the context of most biological redox reactions it is useful to imagine/picture a redox reaction as a simple exchange of electrons between two molecules, an electron donor and an electron acceptor that accepts electrons from the donor.
An analogy with kiwi fruit: To help build this mental picture we offer an analogy. Two people are standing next to one another. At the start, one person is holding a kiwi fruit in their hand and the second person's hands are empty. In this reaction, person 1 gives the kiwi to person 2. At the end of the reaction, person 2 is holding a kiwi and person 1 is not. We can write this exchange of kiwi fruit in the form of a chemical reaction:
person 1(kiwi) + person 2() <-> person 1() + person 2(kiwi).
start/initial state <-> final/end state
If we read this "reaction" from left to right, person 1 is a kiwi donor and person 2 is a kiwi acceptor. We can extend this analogy a little by proposing that person 1 and person 2 have different desire and ability to grab and hold kiwi fruit - we'll call that property "kiwi-potential". We can then propose a situation where person 1 and person 2 compete for a kiwi. Let's propose that person 2 has a higher "kiwi-potential" than person 1 - that is, person 2 has a stronger desire and ability to grab and hold kiwi than person 1.
If we set up a competition where person 1 starts with the kiwi and person 2 competes for it, we should expect that after some time the kiwi will be exchanged to person 2 and stay there most often. At the end of the reaction the kiwi will be with person 2. Due to the difference in "kiwi-potential" between person 1 and person 2, we can say that the spontaneous direction of kiwi flow is from person 1 to person 2. If we ever observed the kiwi flow from person 2 to person 1 we could probably conclude that person 1 required some extra help/energy to make that happen - flow from person 2 to person 1 would be non-spontaneous.
Let's call the "kiwi-potential", Kp. In our analogy, Kpperson 1 < Kpperson 2. We can calculate ∆Kp, the difference in Kp between the two people, and that will tell us something about how likely we can expect to see kiwi exchange hands between these two people. The bigger the difference in Kp the more likely the kiwi will move from the person who has a lower Kp to the person who has the higher Kp.
By definition, to calculate ∆Kp we obtain the solution to ∆Kp = Kpfinal/end - Kpinitial/start. Since the kiwi is with person 2 at the end of the reaction and it starts with person 1 at the beginning of the reaction we would calculate ∆Kp = Kpperson 2 - Kpperson 1.
Doing it with electrons instead of kiwi fruit: To find ∆E0' for a redox reaction we can translate this analogy to the molecular space. Instead of people, we have two molecules. Instead of a kiwi, we have electrons. Different molecules have different inherent abilities to grab and hold electrons and this can be measured by the value E. If two molecules exchange one or more electrons we can imagine that electrons will flow spontaneously from a molecule with lower E0' to one with a higher E0'. We can write a familiar reaction with those substitutions.
molecule 1(electron) + molecule 2() <-> person 1() + molecule 2(electron).
start/initial state <-> final/end state
To find ΔE0', you solve for ΔE0' = E0'-final/end - E0'-initial/start. Alternatively, you can think of it as ΔE0' = E0'-acceptor - E0'-donor.
When evaluating a redox reaction for E0' you therefore need to:
-
First, find which of the reactants is the electron donor. The donor can also be associated with the initial state because it is the molecule that initially (before the start of the reaction) has the electron(s) to donate. This will always be one of the reactants and will be the molecule that gets oxidized (i.e. the molecule that loses electrons).
-
Second, find which of the reactants is the electron acceptor. This will also always be a reactant and will be the molecule that becomes reduced by the reaction (i.e. gains electrons). This molecule can be also associated with the final state since, in its reduced form, it is the molecule that has the electrons at the end of the reaction.
-
Third, calculate ΔE0' = E0'-acceptor - E0'-donor or if you prefer, ΔE0' = E0'-final/end - E0'-initial/start.
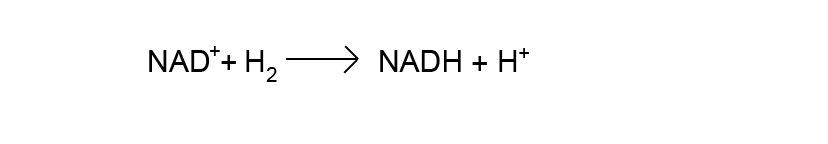
In the example above, we can examine the reactants and determine that NAD+ is the oxidized form of the electron carrier - it can, therefore, not be the donor. This means that H2 must act as the electron donor in this reactant. During the reaction electrons flow onto NAD+ from the donor H2 creating the reduced product NADH and oxidized product H+. To calculate ∆E0' we say that at the start of the reaction the exchanged electrons are on the donor H2. We say that at the end of the reaction the electrons are found on NADH. Calculating ∆E0'requires us to evaluate the difference:
E0'-acceptor - E0'-donor
or equivalently,
E0'-final/end - E0'-initial/start.
Using a redox table to find E0' values for the start and end molecules shows us that NAD+/NADH has an E0' of -0.30 while H+/H2 has an E0' of -0.42.
Therefore, ΔE0' = (-0.30) - (-0.42) = 0.12 V.
We can see intuitively that this reaction is spontaneous: electrons are flowing from a molecule that "wants" electrons less (E0' of H+/H2 = -0.42) to a molecule that wants them more (E0' of NAD+/NADH = -0.30).
Reading Different Looking Redox Towers
Novice students of redox chemistry will all undoubtedly run across different ways of representing a redox tower. These different representations may look different but contain the same information. Without explanation, however, reading these tables - when they look different - can be confusing. We will compare and contrast different common forms of redox towers.
Redox Tower: Type 1

Figure 1. A Generic redox tower with oxidized/reduced couple listed with its reduction potential (E0') .
Attribution: Caidon Iwuagwu
In this type of redox tower, the oxidized and reduced forms of a molecule are separated by a slash. There is a line drawn from each half-reaction to its redox potential E0' reported on the vertical axis.
Redox Tower: Type 2
|
Electron Acceptor |
Electron Donor |
E0' (eV) |
|
CO2 + 24e- → |
glucose |
- 0.43 |
|
2H+ + 2e- → |
H2 |
- 0.42 |
|
CO2 + 6e- → |
methanol |
- 0.38 |
|
NAD+ + 2e- → |
NADH |
- 0.32 |
|
CO2 + 8e- → |
acetate |
- 0.28 |
|
S0 + 2e- → |
H2S |
- 0.28 |
|
SO42- + 8e- → |
H2S |
- 0.22 |
|
Pyruvate + 2e- → |
lactate |
- 0.19 |
|
S4O62- + 2e- → |
S2O32- |
+ 0.024 |
|
Fumarate + 2e- → |
succinate |
+ 0.03 |
|
Cytochrome box + 1e- → |
Cytochrome bred |
+ 0.035 |
|
Ubiquinoneox + 2e- → |
Ubiquinonered |
+ 0.11 |
|
Fe3+ + 1e- → (pH 7) |
Fe2+ |
+ 0.2 |
|
Cytochrome cox + 1e- → |
Cytochrome cred |
+ 0.25 |
|
Cytochrome aox + 1e- → |
Cytochrome ared |
+ 0.39 |
|
NO3- + 2e- → |
NO2- |
+ 0.42 |
|
NO3- + 5e- → |
1/2 N2 |
+ 0.74 |
|
Fe3+ + 1e- → (pH 2) |
Fe2+ |
+ 0.77 |
|
1/2 O2 + 2e- → |
H2O |
+ 0.82 |
In this type of redox tower, each row consists of a half-reaction. The oxidized form of a molecule is shown in the first column, the reduced form of the molecule is shown in the second column. Finally, the E0' value of the molecule is listed in the third column from the left. The number of electrons transferred to reduce the oxidized form of the molecule is shown in column 1. While the format of the table looks different from Type 1 tower, both contain the exact same information.
Redox Tower: Type 3
|
oxidized form |
reduced form |
n (electrons) |
Eo´ (volts) |
|---|---|---|---|
|
CO2 |
glucose |
24 |
-0.43 |
|
2H+ |
H2 |
2 |
-0.42 (at [H+] = 10-7; pH=7) Note: at [H+] = 1; pH=0 the Eo' for hydrogen is ZERO. You will see this in chemistry class. |
|
CO2 |
methanol |
6 |
-0.38 |
|
NAD+ + 2H+ |
NADH + H+ |
2 |
-0.32 |
|
CO2 |
acetate |
8 |
-0.28 |
|
S0 |
H2S |
2 |
-0.28 |
|
SO42- |
H2S |
8 |
-0.22 |
|
Pyruvate + 2H+ |
lactate |
2 |
-0.19 |
| S4O62- | S4O62- | 2 | 0.024 |
|
Fumarate |
succinate |
2 |
0.03 |
| Cytochrome box | Cytochrome bred | 1 | 0.035 |
|
Ubiquinone; (ox) |
Ubiquinone; (red) |
2 |
0.1 |
|
Fe3+ (pH = 7) |
Fe2+ (pH = 7) |
1 |
0.20 |
|
Cytochrome c; Fe3+ |
Cytochrome c; Fe2+ |
1 |
0.25 |
|
Cytochrome a |
Cytochrome a |
1 |
0.39 |
|
Nitrate |
nitrite |
2 |
0.42 |
|
Nitrate |
1/2 N2 |
5 |
0.74 |
|
Fe3+ (pH = 2) |
Fe2+ (pH = 2) |
1 |
0.77 |
|
1/2 O2 + 2H+ |
H2O |
2 |
0.816 |
In this redox tower, the oxidized form of a molecule is in the leftmost column, its reduced form is in the second column from the left, the number of electrons transferred is in the third column from the left, and the E0' is in the far right column.
Again, all of these towers contain the exact same information and are used in an identical manner.
Special note: If you have studied redox chemistry in a formal chemistry course, you might notice two key differences between the towers you use in a biology setting and those used by chemists.
1. In chemistry, the redox towers are flipped relative to those in biology: In chemistry, the molecules with the most positive E0' are listed starting at the top of the table and the compounds with the most negative E0' are listed at the bottom. In bioloigal redox tables molecules with the largest E0' are listed at the bottom while those with the smallest E0' are listed starting at the top. The biology orientation has the advantage of making it easy to picture electrons spontaneously falling down the table from molecules that "want" the electrons less (lower E0') to molecules that "want" electrons more (higher E0').
2. In chemistry, the redox potential for hydrogen (H+/H2) is listed as 0. This is because (a) redox potentials for chemistry are measured under a set of non-biologically relevant standard conditions and (b) hydrogen is being used as the common standard redox potential against which all other redox potentials are measured. In biology, the redox potential for hydrogen (H+/H2) is listed as -0.42. This difference between the chemistry and biology tables comes about because the redox potential for (H+/H2) in biology is measured at a physiological pH of 7.0.
Familiarize yourself with how to read and interpret all three types of redox towers!
Chemistry and Biology Teach Redox Differently
For students who have been taught redox chemistry in a formal chemistry course, biological lessons in redox can sometimes seem like they're talking about something completely different. Not surprisingly, chemists tend to teach the most proper and universally applicable approach to evaluating redox reaction. This approach consists of using a set of rules to formally evaluate whether atoms in a molecule have undergone a change in oxidation state. Meanwhile, biologists tend to approach the discussion of redox reactions by thinking about electron transfers between molecules. It turns out that the approach biologists take is not as rigorous as how chemists approach redox reactions and can sometimes not identify bona fide redox reactions that wouldn't be missed using the chemist's approach. However, since the vast majority of biological redox reactions do involve a transfer of electron (and therefore change in redox states).
Let's look at a specific example to see the differences in approach.
The Chemistry Approach (oxidation numbers):
To evaluate/solve redox reactions in CHEMISTRY, we use the concept of oxidation states. The oxidation state of an element refers to how the electrons are shared between atoms in a chemical compound and they tell us about the movement of the electrons in the redox reaction. There are specific rules to assigning oxidation states, we will not be going over all of them since they will not be applicable to the redox reactions you will see in General Biology, but here are a few:
-
A single element has an oxidation state of 0
-
Fluorine ALWAYS has an oxidation state of -1
-
Hydrogen has an oxidation state of +1 with nonmetals and -1 with metals.
etc.
For more on calculating oxidation states see: <https://chem.libretexts.org/Bookshel...ation_Numbers)>
So, to find which elements are reduced/oxidized when given a redox reaction, you must track the change of the oxidation states between the reactants and the products. Here is an example:
In the unbalanced reaction NO3-+ FADH2 ⟶ NO2-+ FAD+
-
Using the rules, we observe that in NO3-, the oxidation number of Nitrogen is +5. In NO2-, the oxidation number of Nitrogen is +3. So because +5 ⟶ +3, N is reduced in this reaction.
-
We could conduct a similar calculation for key atoms on FAD+ and FADH2 to discover that FADH2 is oxidized in the reaction.
The Biology/Biochemistry Approach (electron flow):
To evaluate/solve redox reactions in biology/biochemistry, we typically do not use or assign oxidation numbers to evaluate redox reactions. Rather we follow the exchange of electrons between molecules. Fortunately, biology tends to reuse a limited number of electron carriers and redox towers tell us which form of a compound is reduced or oxidized. As mentioned above, the basic approach to redox in biology is to define oxidation as: the loss of electrons. Reduction is defined as: the gain of electrons. Here is an example:
NO3-+ FADH2 ⟶ NO2-+ FAD+
Here we examine the reactants and immediately spot the common electron carrier FADH2, the reduced form of the electron carrier. In the products we observe the oxidized form of the electron carrier FAD+. We conclude that FADH2 lost electrons (became oxidized) in the reaction. Since the electrons had to go somewhere they were likely accepted by NO3- which then became reduced to NO2-. In this case the biologist's model arrives at the same conclusion as the chemist's approach through a more intuitive approach that doesn't require memorizing numerous rules and how to apply them.
In our General Biology class, we take the biology/biochemistry approach to redox. You will not need to know how to calculate oxidation states in this course.
DISCLAIMER: DO NOT WORRY IF YOU HAVE NOT TAKEN CHEMISTRY YET !! WE WILL NOT BE USING THE CHEMISTRY APPROACH WHEN IT COMES TO REDOX REACTIONS IN OUR CLASS. THE PURPOSE OF THIS IS JUST TO DISTINGUISH AND HOPEFULLY CLARIFY THE TWO APPROACHES FOR STUDENTS THAT MAY HAVE ALREADY TAKEN A CHEMISTRY COURSE!!

