Bis2A_Singer_Electron_Transport_Chains
- Page ID
- 69315
Electron Transport Chains
An electron transport chain, or ETC,
ETC overview
Step 1: Electrons enter the ETC from an electron donor, such as NADH or FADH2, which
Step 2: After the first red/ox reaction, the initial electron donor will become oxidized and the electron acceptor will become reduced. The difference in red/
Step 3:
Step 4: After usually multiple red/ox transfers,
Note: NADH AND FADH2 ARE NOT THE ONLY ELECTRON DONORS
Electrons entering the ETC do not have to come from NADH or FADH2. Many other compounds can serve as electron donors; the only requirements are (1) that there is an enzyme that can oxidize the electron donor and then reduce another compound, and (2) that the ∆E0' is positive (e.g., ΔG<0). Even a small amount of free energy transfers can add up. For example, there are bacteria that use H2 as an electron donor. This is not too difficult to believe because the half reaction 2H+ + 2
What are the complexes of the ETC?
ETCs comprise a series (at least one) of membrane-associated red/ox proteins or (some are integral) protein complexes (complex =
How do ETC complexes transfer electrons?
As previously mentioned,
Note
This use of prosthetic groups by members of ETC is true for
The electron and proton carriers
- Flavoproteins (Fp), these proteins contain an organic prosthetic group called a flavin, which is the actual moiety that undergoes the oxidation/reduction reaction. FADH2 is an example of
an Fp . - Quinones are a family of lipids, which means they are soluble within the membrane.
- We also note that
we consider NADH and NADPH electron (2e-) and proton (2 H+) carriers.
Electron carriers
- Cytochromes are proteins that contain a heme prosthetic group. The heme can carry a single electron.
- Iron-Sulfur proteins contain a nonheme iron-sulfur cluster that can carry an electron. We often abbreviate the prosthetic group as Fe-
S
Introduction to Mobile Energy Carriers
Section Summary
Energy moves and transfers within the cell in a variety of ways. One critical mechanism that nature has developed is the use of recyclable molecular energy carriers. While there are several major recyclable energy carriers, they all share some common functional features:
Properties of Key Cellular Molecular Energy Carriers
- We think of the energy carriers as existing in "pools" of
available carriers. One could, by analogy, consider these mobile energy carriers analogous to the delivery vehicles of parcel carriers—the company has a certain "pool" ofavailable vehicles at any one time to pickup and make deliveries. - Each individual carrier in the pool can exist in one of multiple distinct states: it is
either carrying a "load" of energy, a fractional load, or is "empty". The molecule caninterconvert between "loaded" and empty and thus canbe recycled . Again by analogy, the delivery vehicles can beeither carrying packages or be empty and switch between these states. - The balance or ratio in the pool between "loaded" and "unloaded" carriers is important for cellular function, is regulated by the cell, and can often tell us something about the state of a cell. Likewise, a parcel carrier service keeps close tabs on how full or empty their delivery vehicles are—if they are too full, there may be insufficient "empty" trucks to pick up new packages; if they are too empty, business must not be going well or they shut it down. There is an appropriate balance for different situations.
In this course, we will examine two major types of molecular recyclable energy carriers: (1) the adenine nucleotides: nicotinamide adenine dinucleotide (NAD+), a close relative, nicotinamide adenine dinucleotide phosphate (NADP+), and flavin adenine dinucleotide (FAD2+) and (2) nucleotide mono-, di-, and triphosphates, with particular attention paid to adenosine triphosphate (ATP). These two types of molecules participate in a variety of energy transfer reactions. We primarily associate adenine nucleotides with red/ox chemistry, and nucleotide triphosphates with energy transfers linked to the hydrolysis or condensation of inorganic phosphates.
Red/ox chemistry and electron carriers
The oxidation of, or removal of an electron from, a molecule (whether accompanied with the removal of an accompanying proton or not) results in a change of free energy for that molecule—matter, internal energy, and entropy have all changed. Likewise, the reduction of a molecule also changes its free energy. The magnitude of change in free energy and its direction (positive or negative) for a red/ox reaction dictates the spontaneity of the reaction and how much energy it transfers. In biological systems, where a great deal of energy transfer happens via red/ox reactions, it is important to understand how these reactions
Note: BURNING BEAR
Relate the burning of (the full oxidation of the sugar in) a gummy bear with the last paragraph above. What does that demonstration have to do with our upcoming discussion on red/ox carriers? There is some mention above already—can you find it?
Note: DESIGN CHALLENGE
The problem alluded to in the previous discussion question is a great place to bring in the design challenge rubric. If you recall, the first step of the rubric asks that you define a problem or question. Here, let's imagine that there is a problem to define for which the mobile electron carriers below helped Nature solve.
***Remember, evolution DOES NOT forward-engineer solutions to problems, but in retrospect, we can use our imagination and logic to infer that what we see preserved by natural selection provided a selective advantage, because the natural innovation "solved" a problem that limited success.***
Design challenge for red/ox carriers
- What was a problem
( s) that the evolution of mobile electron carriers helped solve? - The next step of the design challenge asks you to identify criteria for successful solutions. What are criteria for success in the problem you've identified?
- Step 3 in the design challenge asks you to identify solutions. Well, here Nature has identified some for us—we consider three in the reading below. It looks like Nature is happy to have multiple solutions to the problem.
- The penultimate step of the design challenge rubric asks you to test the proposed solutions against the criteria for success. This should make you think/discuss why there are multiple different electron carriers. Are there different criteria for success? Are they each solving slightly different problems? What do you think? Be on the lookout as we go through metabolism for clues.
NAD+/H and FADH/H2
In living systems, a small class of compounds function as electron shuttles: they bind and carry electrons between compounds in different metabolic pathways. The principal electron carriers we will consider derive from the B vitamin group and nucleotides. These compounds can both become reduced (that is, they accept electrons) or oxidized (they lose electrons) depending on the reduction potential of a potential electron donor or acceptor that they might transfer electrons to and from. Nicotinamide adenine dinucleotide (NAD+) (we show the structure below) derives from vitamin B3, niacin. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron).
We are expecting you to memorize the two forms of NAD+/NADH, know which form is oxidized and which is reduced, and be able to recognize either form on the spot in a chemical reaction.
NAD+ can accept electrons from an organic molecule according to the general equation:

Here is some vocabulary review: when electrons are added to a compound, we say the compound has been reduced. A compound that reduces (donates electrons to) another is called a reducing agent. In the above equation, RH is a reducing agent, and NAD+ becomes reduced to NADH. When electrons leave a compound, it becomes oxidized. A compound that oxidizes another is called an oxidizing agent. In the above equation, NAD+ is an oxidizing agent, and RH is oxidized to R. Put another way, the reducing agent gets oxidized and the oxidizing agent gets reduced.
You need to get this down! We will (a) test specifically on your ability to do so (as "easy" questions), and (b) we will use the terms with the expectation that you know what they mean and can relate them to biochemical reactions correctly (in class and on tests).
You will also encounter a second variation of NAD+, NADP+. It is structurally very similar to NAD+, but it contains an extra phosphate group and plays an important role in anabolic reactions, such as photosynthesis. Another nucleotide-based electron carrier that you will also encounter in this course and beyond, flavin adenine dinucleotide (FAD+), derives from vitamin B2, also called riboflavin. Its reduced form is FADH2. Learn to recognize these molecules as electron carriers.

Figure 1. The oxidized form of the electron carrier (NAD+) is shown on the left, and the reduced form (NADH) is shown on the right. The nitrogenous base in NADH has one more hydrogen ion and two more electrons than in NAD+.
The cell uses NAD+ to "pull" electrons off of compounds and to "carry" them to other locations within the cell; thus we call it an electron carrier. Many metabolic processes we will discuss in this class involve NAD(P)+/H compounds. For example, in its oxidized form, NAD+ is used as a reactant in glycolysis and the TCA cycle, whereas in its reduced form (NADH), it is a reactant in fermentation reactions and electron transport chains (ETC). We will discuss each of these processes in later modules.
Energy story for a red/ox reaction
***As a rule of thumb, when we see NAD+/H as a reactant or product, we know we are looking at a red/ox reaction.***
When NADH is a product and NAD+ is a reactant, we know that NAD+ has become reduced (forming NADH); therefore, the other reactant must have been the electron donor and become oxidized. The reverse is also true. If NADH has become NAD+, then the other reactant must have gained the electron from NADH and become reduced.
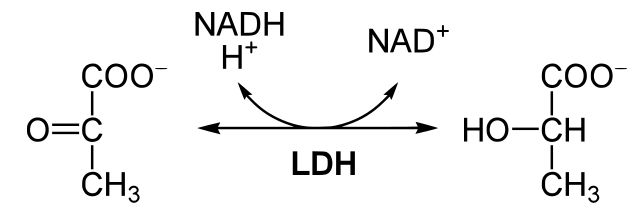
Figure 2. This reaction shows the conversion of pyruvate to lactic acid coupled with the conversion of NADH to NAD+. Source: https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/sequential_reactions
In the figure above, we see pyruvate becoming lactic acid, coupled with the conversion of NADH into NAD+. LDH catalyses this reaction. Using our "rule of thumb" above, we categorize this reaction as a red/ox reaction. NADH is the reduced form of the electron carrier, and NADH is converted into NAD+. This half of the reaction results in the oxidation of the electron carrier. Pyruvate is converted into lactic acid in this reaction. Both sugars are negatively charged, so it would be difficult to see which compound is more reduced using the charges of the compounds. However, we know that pyruvate has become reduced to form lactic acid, because this conversion is coupled to the oxidation of NADH into NAD+. But how can we tell that lactic acid is more reduced than pyruvate? The answer is to look at the carbon-hydrogen bonds in both compounds. As electrons transfer, they are often accompanied by a hydrogen atom. Pyruvate has a total of three C-H bonds, while lactic acid has four C-H bonds. When we compare these two compounds in the before and after states, we see that lactic acid has one more C-H bond; therefore, lactic acid is more reduced than pyruvate. This holds true for multiple compounds. For example, in the figure below, you should be able to rank the compounds from most to least reduced using the C-H bonds as your guide.

Figure 3. Above are a series of compounds than can be ranked or reorganized from most to least reduced. Compare the number of C-H bonds in each compound. Carbon dioxide has no C-H bonds and is the most oxidized form of carbon we will discuss in this class. Answer: the most reduced is methane (compound 3), then methanol (4), formaldehyde (1), carboxylic acid (2), and finally carbon dioxide (5).

Figure 4. This reaction shows the conversion of G3P, NAD+, and Pi into NADH and 1,3-BPG. This reaction is catalyzed by glyceraldehyde-3-phosphate dehydrogenase.
Energy story for the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase:
Lets make an energy story for the reaction above.
First, let us characterize the reactants and products. The reactants are glyceraldehyde-3-phosphate (a carbon compound), Pi (inorganic phosphate), and NAD+. These three reactants enter a chemical reaction to produce two products, NADH and 1,3-bisphosphoglycerate. If you look closely, you can see that the 1,3-BPG contains two phosphates. This is important since a chemical reaction should lose no mass between its beginning and its end. There are two phosphates in the reactants, so there must be two phosphates in the products (conservation of mass!). You can double check the book keeping of mass for all other atoms. It should also tabulate correctly. An enzyme called glyceraldehyde-3-phosphate dehydrogenasethat catalyzes this reaction. The standard free energy change of this reaction is ~6.3 kJ/mol, so under standard conditions, we can say that the free energy of the products is higher than that of the reactants and that this reaction is not spontaneous under standard conditions.
What can we say about this reaction when it is catalyzed by glyceraldehyde-3-phosphate dehydrogenase?
This is a red/ox reaction. We know that because we have produced a reduced electron carrier (NADH) as a product and NAD+ is a reactant. Where did the electron come from to make NADH? The electron must have come from the other reactant (the carbon compound).
Aerobic versus anaerobic respiration
We humans use oxygen as the terminal electron acceptor for the ETCs in our cells. This is also the case for many of the organisms we intentionally and frequently interact with (e.g. our classmates, pets, food animals, etc). We breathe in oxygen; Our cells take it up and transport it into the mitochondria, where it becomes the final acceptor of electrons from our electron transport chains. We call the process where oxygen
While we may use oxygen as the terminal electron acceptor for our respiratory chains, this is not the only mode of respiration on the planet. The more general processes of respiration evolved when oxygen was not a major component of the atmosphere. As a result, many organisms can use a variety of compounds, including nitrate (NO3-), nitrite (NO2-), even iron (Fe3+) as terminal electron acceptors. When oxygen is NOT the terminal electron acceptor, we refer the process to as anaerobic respiration. Therefore, respiration or oxidative phosphorylation does not require oxygen at all; It requires a compound with a high enough reduction potential to act as a terminal electron acceptor, accepting electrons from one complex within the ETC.
The ability of some organisms to vary their terminal electron acceptor provides metabolic flexibility and can ensure better survival if any given terminal acceptor is in limited supply. Think about this: in the absence of oxygen, we die; but other organisms can use a different terminal electron acceptor when conditions change to survive.
Possible NB Discussion  Point
Point
Nature has figured out how to use different molecules as terminal electron acceptors of ETCs. Yet humans
A generic example: A simple, two-complex ETC
The figure below depicts a generic electron transport
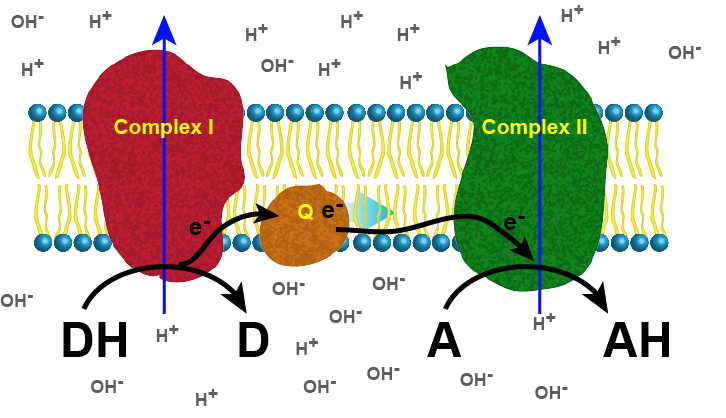
Figure 1. Generic 2 complex electron transport chain. In the figure, DH is the electron donor (donor reduced), and D is the donor oxidized.
Attribution:
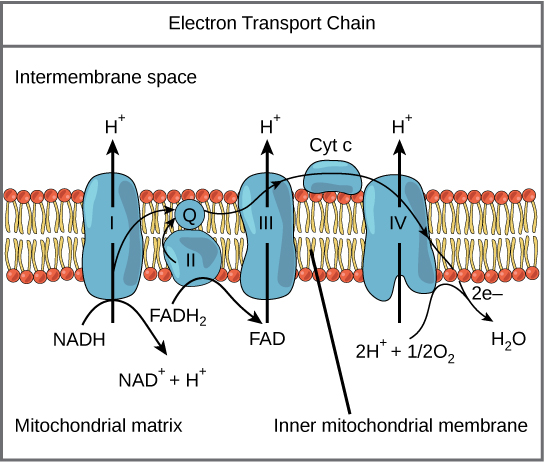
Detailed look at aerobic respiration
The eukaryotic mitochondria have evolved a very efficient ETC. There are four complexes composed of proteins, labeled I
Figure 2. The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen.
Complex I
To start, NADH delivers two electrons to the first protein complex. This complex, labeled I in Figure 2, includes flavin mononucleotide (FMN) and iron-sulfur (Fe-S)-containing proteins. FMN, which is derived from vitamin B2, also called riboflavin, is one of several prosthetic groups or cofactors in the electron transport chain. Prosthetic groups are organic or inorganic, nonpeptide molecules bound to a protein that facilitate its function; prosthetic groups include coenzymes, which are the prosthetic groups of enzymes. We also call the enzyme in Complex I NADH dehydrogenase. This protein complex contains 45 individual polypeptide chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space helping to generate and maintain a hydrogen ion gradient between the two compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives FADH2, which does not pass through Complex I. The compound connecting the first and second complexes to the third is ubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once reduced, (QH2), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from Complex I and the electrons derived from FADH2 from Complex II, succinate dehydrogenase. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules
Complex III
The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; we also call this complex cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is like the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe2+ (reduced) and Fe3+ (oxidized). The heme molecules in the cytochromes have slightly different characteristics because of the effects of the different proteins binding them, giving slightly different characteristics to each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a time).
Complex IV
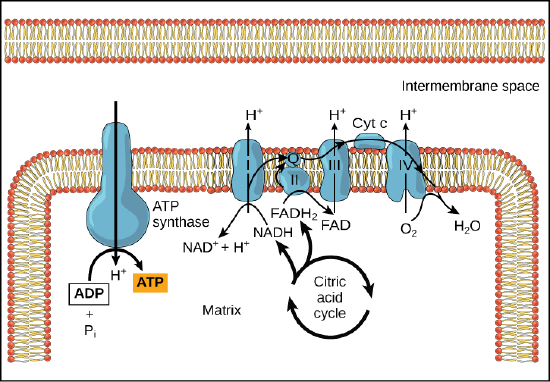
Chemiosmosis
In
If the membrane were open to diffusion by protons, the ions would
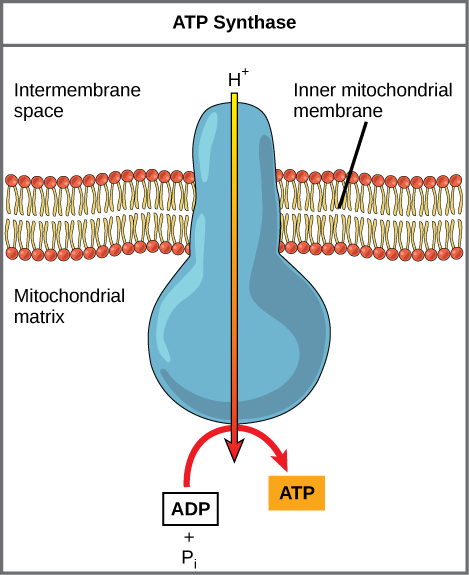
Figure
Credit:
Possible NB Discussion  Point
Point
Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you expect the pH of the
In healthy cells,
Figure 4. In oxidative phosphorylation,
Helpful link: