Light Energy and Pigments*#
- Page ID
- 21306
Light Energy and Pigments
Light Energy
The sun emits an enormous amount of electromagnetic radiation (solar energy) that spans a broad swath of the electromagnetic spectrum, the range of all radiation frequencies. When solar radiation reaches Earth, a fraction of this energy interacts with and may transfer to the matter on the planet. This energy transfer results in a wide variety of different phenomena, from influencing weather patterns to driving myriad biological processes. In BIS2A, we
First, however we need to refresh a few key properties of light:
- Light in a vacuum travels at a constant speed of 299,792,458
m /s. We often abbreviate the speed of light with the variable "c ". - Light has properties of waves. A specific "color" of light has a characteristic wavelength.
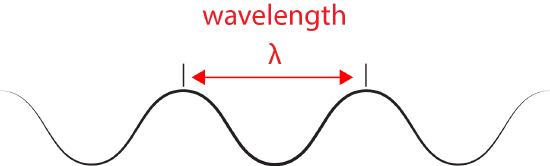
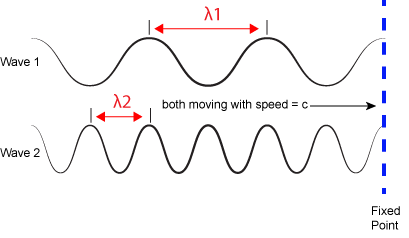
We refer to the distance between peaks in a wave as the wavelength and abbreviate it with the Greek letter lambda (Ⲗ). Attribution:
The inverse proportionality of frequency and wavelength. Wave 1 has a wavelength that is 2x that of wave 2 (Ⲗ1 > Ⲗ2). If the two waves are traveling at the same speed (c)—imagine that both of the whole lines that are dragged past the fixed vertical line at the same speed—then the number of times a wave peak passes a fixed point is greater for wave 2 than wave 1 (f2 > f1). Attribution: Marc T. Facciotti (original work)
3. Finally, each frequency (or wavelength) of light is associated with a specific energy. We'll call energy "E". The relationship between frequency and energy is:
\[E = h \times f\]
where h is a constant called the Planck constant (~6.626x10-34 Joule•second when frequency is expressed in cycles per second). Given the relationship between frequency and wavelength, you can also write E = h*c/Ⲗ. Therefore, the larger the frequency (or shorter the wavelength), the more energy is associated with a specific "color". Wave 2 in the figure above is associated with greater energy than wave 1.
The sun emits energy in the form of electromagnetic radiation. All electromagnetic radiation, including visible light, is characterized by its wavelength. The longer the wavelength, the less energy it carries. The shorter the wavelength, the more energy is associated with that band of the electromagnetic spectrum.
The Light We See
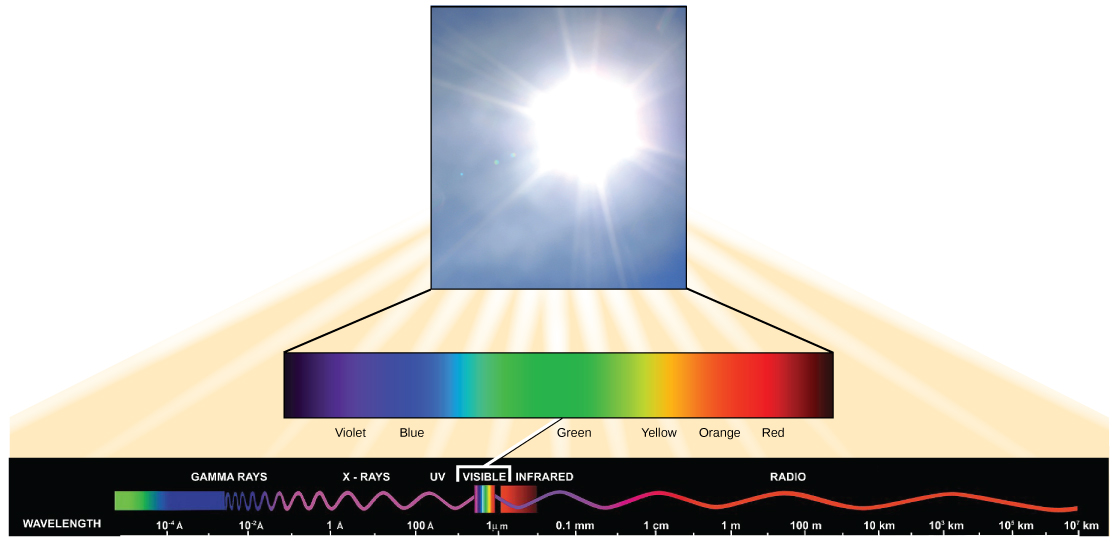
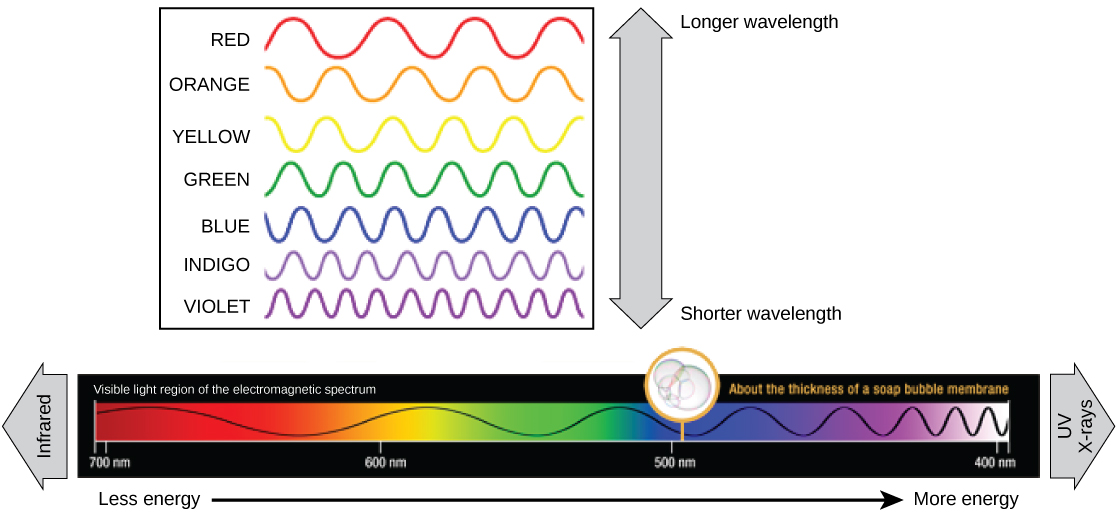
The visible light seen by humans as white light is composed of a rainbow of colors, each with a characteristic wavelength. Certain objects, such as a prism or a drop of water, disperse white light to reveal the colors to the human eye. In the visible spectrum, violet and blue light have shorter (higher energy) wavelengths while the orange and red light have a longer (lower energy) wavelengths.
The colors of visible light do not carry the same amount of energy. Violet has the shortest wavelength and therefore carries the most energy, whereas red has the longest wavelength and carries the least amount of energy. Credit: modification of work by NASA
Absorption by Pigments
The interaction between light and biological systems occurs through several mechanisms, some of which you may learn about in upper division courses in cellular physiology or biophysical chemistry. In BIS2A, we concern ourselves mostly with the interaction between light and biological pigments. These interactions can start a variety of light-dependent biological processes that can be grossly grouped into two functional categories: cellular signaling and energy harvesting. Signaling molecules perceive changes in the environment (in this case, changes in light). An example of a signaling interaction might be the interaction between light and the pigments expressed in an eye. Light/pigment interactions that are involved in energy harvesting are used for—not surprisingly—capturing the energy in the light and transferring it to the cell to fuel biological processes. Photophosphorylation, which we will learn more about soon, is one example of an energy harvesting interaction.
Possible NB Discussion  Point
Point
Photophosphorylation is a process involving an electron transport chain that allows organisms to harvest energy from light. Some of you may already be familiar with this process. Many of you are learning about this for the first time. Given your current knowledge base, offer your best explanation or hypothesis as to how light interacts with the ETC. You will have a chance to revisit this topic very soon.
At the center of the biological interactions with light are groups of molecules we call organic pigments. Whether in the human retina, chloroplast thylakoid, or microbial membrane, organic pigments often have specific ranges of energy or wavelengths that they can absorb. The sensitivity of these molecules for different wavelengths of light is due to their unique chemical makeups and structures. A range of the electromagnetic spectrum is given a couple of special names because of the sensitivity of some key biological pigments: The retinal pigment in our eyes, when coupled with an opsin sensor protein, “sees” (absorbs) light predominantly between the wavelengths between of 700 nm and 400 nm. Because this range defines the physical limits of the electromagnetic spectrum that we can actually see with our eyes, we refer to this wavelength range as the “visible range”. For similar reasons, as plants pigment molecules tend to absorb wavelengths of light mostly between 700 nm and 400 nm, plant physiologists refer to this range of wavelengths as "photosynthetically active radiation".
Three Key Types of Pigments Commonly Discussed in General Biology
Chlorophylls
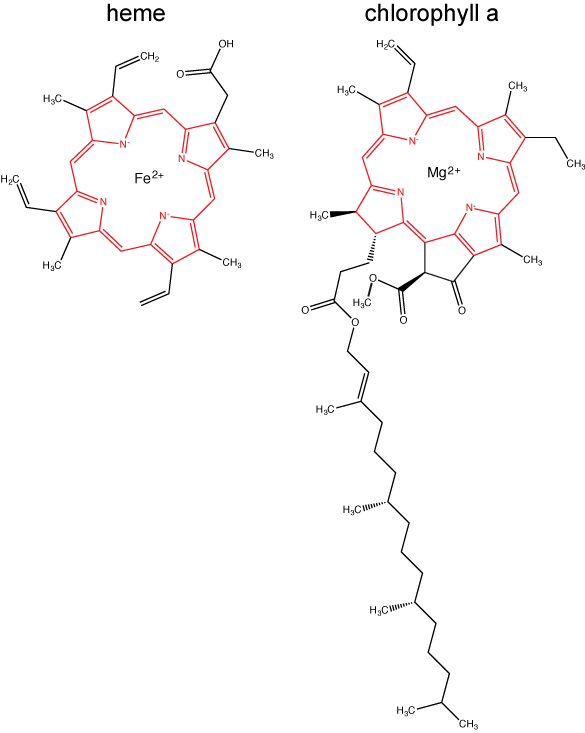
Chlorophylls (including bacteriochlorophylls) are part of a large family of pigment molecules. There are five major chlorophyll pigments named: a, b, c, d, and f. Chlorophyll a is related to a class of more ancient molecules found in bacteria called bacteriochlorophylls. Chlorophylls are structurally characterized by ring-like porphyrin group that coordinates a metal ion. This ring structure is chemically related to the structure of heme compounds that also coordinate a metal and are involved in oxygen binding and/or transport in many organisms. We distinguish different chlorophylls from one another by different "decorations"/chemical groups on the porphyrin ring.
The structure of heme and chlorophyll a molecules. The common porphyrin ring is colored in red. Attribution: Marc T. Facciotti (original work)
Carotenoids
Carotenoids are the red/orange/yellow pigments found in nature. They are found in fruit—the red of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange peel (β-carotene)—which serve as biological "advertisements" to attract seed dispersers (animals or insects that may carry seeds elsewhere). In photosynthesis, carotenoids function as photosynthetic pigments. In addition, when a leaf is exposed to full sun, that surface is required to process an enormous amount of energy; If that energy is not managed properly, it can do significant damage. Therefore, many carotenoids help absorb excess energy in light and safely dissipate that energy as heat.
Flavonoids
Flavonoids are a very broad class of compounds that are found in great diversity in plants. These molecules come in many forms but all share a common core structure shown below. The diversity of flavonoids comes from the many combinations of functional groups that can "decorate" the core flavone.
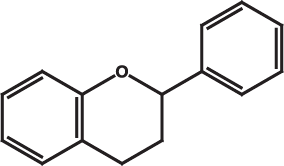
The core ring structure of flavans.
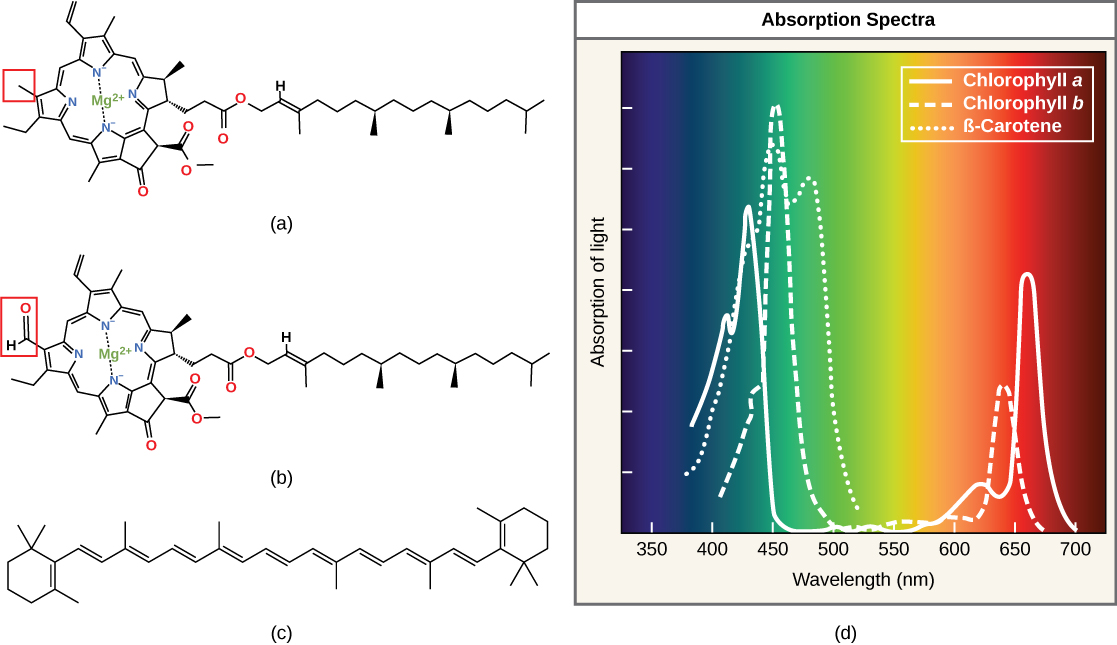
Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light. We define this characteristic as the pigment's absorption spectrum. The graph in the figure below shows the absorption spectra for chlorophyll a, chlorophyll b, and a type of carotenoid pigment called β-carotene (which absorbs blue and green light). Notice how each pigment has a distinct set of peaks and troughs, revealing a highly specific pattern of absorption. These differences in absorbance are because of differences in chemical structure (some of these are highlighted in the figure). Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red), but not green. Because chlorophyll reflects green light and absorbs other wavelengths of light, things containing this pigment appear green. Carotenoids absorb in the short-wavelength blue region and reflect the longer yellow, red, and orange wavelengths.
(a) Chlorophyll a, (b) chlorophyll b, and (c) β-carotene are hydrophobic organic pigments found in the thylakoid membrane. Chlorophyll a and b, which are identical except for the part indicated in the red box, are responsible for the green color of leaves. Note how the small amount of difference in chemical composition between different chlorophylls leads to different absorption spectra. β-carotene is responsible for the orange color in carrots. Each pigment has a unique absorbance spectrum (d).
Importance of having multiple different pigments
Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity and the number of wavelengths decrease and change, respectively, with depth. Other organisms grow in competition for light. For instance, plants on the rainforest floor must be able to absorb any bit of light that comes through because the taller trees absorb most of the sunlight and scatter the remaining solar radiation. To account for these variable light conditions, many photosynthetic organisms have a mixture of pigments whose expression they can tune to improve the organism's ability to absorb energy from a wider range of wavelengths than would be possible with one pigment alone.