S2019_Lecture_18_Reading
- Page ID
- 22143
Light Independent Reactions and Carbon Fixation
A short introduction
The general principle of carbon fixation is that some cells under certain conditions can take inorganic carbon, CO2 (also referred to as mineralized carbon), and reduce it to a usable cellular form. Most of us are aware that green plants can take up CO2 and produce O2 in a process known as photosynthesis. We have already discussed photophosphorylation, the ability of a cell to transfer light energy onto chemicals and ultimately to produce the energy carriers ATP and NADPH in a process known as the light reactions. In photosynthesis, the plant cells use the ATP and NADPH formed during photophosphorylation to reduce CO2 to sugar, (as we will see, specifically G3P) in what are called the dark reactions. While we appreciate that this process happens in green plants, photosynthesis had its evolutionary origins in the bacterial world. In this module we will go over the general reactions of the Calvin Cycle, a reductive pathway that incorporates CO2 into cellular material.
In photosynthetic bacteria, such as Cyanobacteria and purple non-sulfur bacteria, as well plants, the energy (ATP) and reducing power (NADPH) - a term used to describe electron carriers in their reduced state - obtained from photophosphorylation is coupled to "Carbon Fixation", the incorporation of inorganic carbon (CO2) into organic molecules; initially as glyceraldehyde-3-phosphate (G3P) and eventually into glucose. Organisms that can obtain all of their required carbon from an inorganic source (CO2) are referred to as autotrophs, while those organisms that require organic forms of carbon, such as glucose or amino acids, are referred to as heterotrophs. The biological pathway that leads to carbon fixation is called the Calvin Cycle and is a reductive pathway (consumes energy/uses electrons) which leads to the reduction of CO2 to G3P.
The Calvin Cycle: the reduction of CO2 to Glyceraldehyde 3-Phosphate
Figure 1. Light reactions harness energy from the sun to produce chemical bonds, ATP, and NADPH. These energy-carrying molecules are made in the stroma where carbon fixation takes place.
In plant cells, the Calvin cycle is located in the chloroplasts. While the process is similar in bacteria, there are no specific organelles that house the Calvin Cycle and the reactions occur in the cytoplasm around a complex membrane system derived from the plasma membrane. This intracellular membrane system can be quite complex and highly regulated. There is strong evidence that supports the hypothesis that the origin of chloroplasts from a symbiosis between cyanobacteria and early plant cells.
Stage 1: Carbon Fixation
In the stroma of plant chloroplasts, in addition to CO2, two other components are present to initiate the light-independent reactions: an enzyme called ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), and three molecules of ribulose bisphosphate (RuBP), as shown in the figure below. Ribulose-1,5-bisphosphate (RuBP) is composed of five carbon atoms and includes two phosphates.
RuBisCO catalyzes a reaction between CO2 and RuBP. For each CO2 molecule that reacts with one RuBP, two molecules of another compound (3-PGA) form. PGA has three carbons and one phosphate. Each turn of the cycle involves only one RuBP and one carbon dioxide and forms two molecules of 3-PGA. The number of carbon atoms remains the same, as the atoms move to form new bonds during the reactions (3 atoms from 3CO2 + 15 atoms from 3RuBP = 18 atoms in 3 atoms of 3-PGA). This process is called carbon fixation, because CO2 is “fixed” from an inorganic form into an organic molecule.
Stage 2: Reduction
ATP and NADPH are used to convert the six molecules of 3-PGA into six molecules of a chemical called glyceraldehyde 3-phosphate (G3P) - a carbon compound that is also found in glycolysis. Six molecules of both ATP and NADPH are used in the process. The exergonic process of ATP hydrolysis is in effect driving the endergonic redox reactions, creating ADP and NADP+. Both of these "spent" molecules (ADP and NADP+) return to the nearby light-dependent reactions to be recycled back into ATP and NADPH.
Stage 3: Regeneration
Interestingly, at this point, only one of the G3P molecules leaves the Calvin cycle to contribute to the formation of other compounds needed by the organism. In plants, because the G3P exported from the Calvin cycle has three carbon atoms, it takes three “turns” of the Calvin cycle to fix enough net carbon to export one G3P. But each turn makes two G3Ps, thus three turns make six G3Ps. One is exported while the remaining five G3P molecules remain in the cycle and are used to regenerate RuBP, which enables the system to prepare for more CO2 to be fixed. Three more molecules of ATP are used in these regeneration reactions.
Additional Links of Interest
Khan Academy Links
Chemwiki links
YouTube Videos
Introduction into the pentose phosphate pathway
Most introductory biology and biochemistry courses focus on glycolysis (oxidation of glucose to pyruvate) and the TCA cycle (oxidation of pyruvate to acetyl-CoA and the eventual complete oxidation to CO2). While these are extremely important and universal reactions, most courses leave out the pentose phosphate pathway (PPP) or hexose monophosphate shunt. This pathway, like the TCA cycle, is partially cyclic in nature in which three glucose molecules enter and two glucose and one glyceraldyde-3-phosphate (G3P) leave. The two glucose molecules can recycle, and the G3P enters glycolysis. Its an important pathway because it is the primary mechanism for the formation of pentoses, the five-carbon sugar required for nucleotide biosynthesis as well as the formation of a variety of other essential cellular components and NADPH, the cellular reductant primarily used in anabolic reactions.
A note from the Instructor
As with the modules on glycolysis and the TCA cycle, there is a lot of material in this module. As with the other modules, I do not expect you to memorize specific names of compounds or enzymes. However, I will give you those names for completeness. For exams, I will always provide you with the pathways we discuss in class and in the BioStax Biology text modules. What you need to be able to do is understand what is going on in each reaction. We will go over in lecture problems that will be similar to those I will ask of you on exams. Do not be overwhelmed with specific enzyme names and specific structures. What you should know are the general types of enzymes used and the types of structures found. For example, you do not need to memorize the structures of eyrthose or sedoheptulose. You will need to know that both are sugars, the former a four-carbon sugar and the latter a seven-carbon sugar. Remember the ending "ose" identifies the compound as a sugar. In addition, you will not need to know the details of the two unique reactions found in the PPP, the transketolase and transaldolase reactions, though you do need to be able to identify a ketone containing sugar versus an aldehyde containing sugar. Finally, you will not be expected to memorize enzyme names, but like in glycolysis and the TCA cycle, you will be expected to know the various types of reactions a type of enzyme can catalyze, for example, a transaldolase moves aldehyde groups from one compound to another. This is the level of understanding I expect. If you have any questions please ask.
Oxidative pentose phosphate pathway: a.k.a., the hexose monophosphate shunt
While glycolysis has evolved to oxidize hexoses to form carbon precursors for biosynthesis, energy (ATP), and reducing power (NADH), the Pentose Phosphate Pathway (PPP) has evolved to utilize pentoses or five-carbon sugars. Pentoses are required precursors for nucleotides and other essential biomolecules. Instead of NADH, the PPP also generates NADPH which is required for most anabolic reactions. The PPP, in conjunction with glycolysis and the TCA cycle, make up what we call Central Metabolism. These three central pathways (along with the reaction pyruvate to Acetyl-CoA) are responsible for producing all of the necessary precursor molecules required by all cells. The PPP is responsible for producing pentos-phosphates (give carbon sugars), eyrthrose-phosphate (four-carbon sugars), and NADPH. This pathway is also responsible for the production of sedoheptulose-phosphate, an essential seven-carbon sugar used in the outer cell membranes of Gram-negative bacteria.
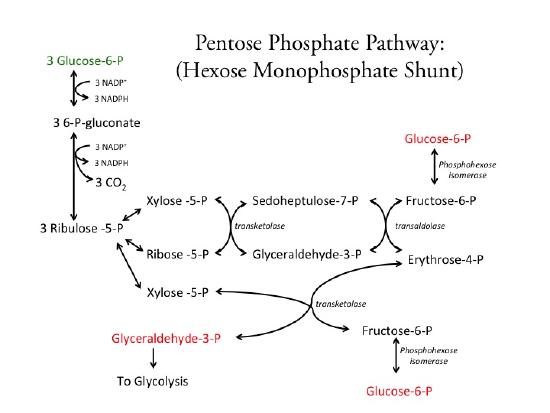
Below is a diagram of the pathway. The pathway is complex and involves a variety of novel rearrangement reactions that move two and three carbon units around. These reactions called transaldolases and transketalases are used to produce the intermediates within the pathway. The net result is oxidation and subsequent decarboxylation of glucose to form a pentose. The total reaction involves three glucose-6-phosphate (in green) molecules being oxidized to form three CO2 molecules, one glyceraldehyde-phosphate (in red), and two hexose-phosphates (in red). In this cycle, the formed glyceradehyde-phosphate feeds into glycolysis and the two hexose-phosphates (e.g., glucose-phosphates) can recycle into the PPP or gycolysis.
As shown in Figure 1, the net result of the pathway is one trios-phosphate (glyceraldehyde-3-phosphate) that can then be further oxidized via glycolysis, two recycled hexose-phosphates (in the form of either glucose-6-phosphate or fructose-6-phosphate), and NADPH which is a required reductant for many biosynthetic (anabolic) reactions. The pathway provides a variety of intermediate sugar-phosphates that the cell may require, such as pentose-phosphates (for nucleotides and some amino acids), erythrose-phosphate (for amino acids) and sedohepulose-phosphate (for gram-negative bacteria).
The PPP along with glycolysis, the TCA cycle, and the oxidation of pyruvate to acetyl-CoA makes up the major pathways of central metabolism and is required to some degree of all organisms to construct the basic substrates to create the building blocks of life.
Section summary
By the end of this module you should be able to describe the role the pentose phosphate pathway plays in central metabolism and determine the end products of the pathway.