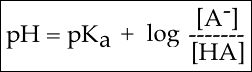S2019_Lecture_04_Reading
- Page ID
- 21449
 pKa
pKa
pKa is defined as the negative log10 of the dissociation constant of an acid, its Ka. Therefore, the pKa is a quantitative measure of how easily or how readily the acid gives up its proton [H+] in solution and thus a measure of the "strength" of the acid. Strong acids have a small pKa, weak acids have a larger pKa.
The most common acid we will talk about in BIS2A is the carboxylic acid functional group. These acids are typically weak acids, meaning that they only partially dissociate (into H+ cations and RCOO- anions) in neutral solution. HCL (hydrogen chloride) is a common strong acid, meaning that it will fully dissociate into H+ and Cl-.
Note that the key difference in the figure below between a strong acid or base and a weak acid or base is the single arrow (strong) versus a double arrow (weak). In the case of the single arrow you can interpret that by imagining that nearly all reactants have been converted into products. Moreover, it is difficult for the reaction to reverse backwards to a state where the protons are again associated with the molecule there were associated with before. In the case of a weak acid or base, the double-sided arrow can be interpreted by picturing a reaction in which:
- both forms of the conjugate acid or base (that is what we call the molecule that "holds" the proton - i.e. CH3OOH and CH3OO-, respectively in the figure) are present at the same time and
- the ratio of those two quantities can change easily by moving the reaction in either direction.
Figure 1. An example of strong acids and strong bases in their protonation and deprotonation states. The value of their pKa is shown on the left. Attribution: Marc T. Facciotti
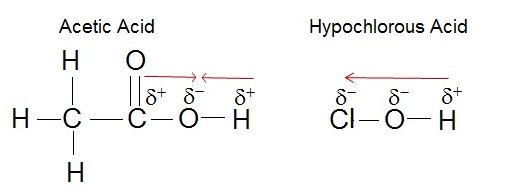
Electronegativity plays a role in the strength of an acid. If we consider the hydroxyl group as an example, the greater electronegativity of the atom or atoms (indicated R) attached to the hydroxyl group in the acid R-O-H results in a weaker H-O bond, which is thus more readily ionized. This means that the pull on the electrons away from the hydrogen atom gets greater when the oxygen atom attached to the hydrogen atom is also attached to another electronegative atom. An example of this is HOCL. The electronegative Cl polarizes the H-O bond, weakening it and facilitating the ionization of the hydrogen. If we compare this to a weak acid where the oxygen is bound to a carbon atom (as in carboxylic acids) the oxygen is bound to the hydrogen and carbon atom. In this case, the oxygen is not bound to another electronegative atom. Thus the H-O bond is not further destabilized and the acid is considered a weak acid (it does not give up the proton as easily as a strong acid).
Figure 2. The strength of the acid can be determined by the electronegativity of the atom the oxygen is bound to. For example, the weak acid Acetic Acid, the oxygen is bound to carbon, an atom with low electronegativity. In the strong acid, Hypochlorous acid, the oxygen atom is bound to an even more electronegative Chloride atom.
Attribution: Erin Easlon
In Bis2A you are going to be asked to relate pH and pKa to each other when discussing the protonation state of an acid or base, for example, in amino acids. How can we use the information given in this module to answer the question: Will the functional groups on the amino acid Glutamate be protonated or deprotonated at a pH of 2, at a pH of 8, at a pH of 11?
In order to start answering this question we need to create a relationship between pH and pKa. The relationship between pKa and pH is mathematically represented by Henderson-Hasselbach equation shown below, where [A-] represents the deprotonated form of the acid and [HA] represents the protonated form of the acid.
Figure 3. The Henderson-Hasselbach equation
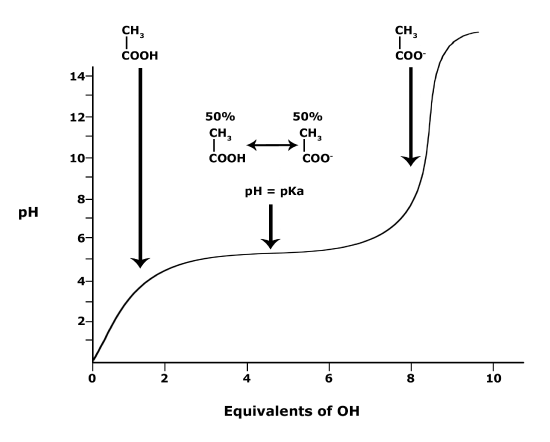
A solution to this equation is obtained by setting pH = pKa. In this case, log([A-] / [HA]) = 0, and [A-] / [HA] = 1. This means that when the pH is equal to the pKa there are equal amounts of protonated and deprotonated forms of the acid. For example, if the pKa of the acid is 4.75, at a pH of 4.75 that acid will exist as 50% protonated and 50% deprotonated. This also means that as the pH rises, more of the acid will be converted into the deprotonated state and at some point the pH will be so high that the majority of the acid will exist in the deprotonated state.
Figure 4. This graph depicts the protonation state of acetic acid as the pH changes. At a pH below the pKa, the acid is protonated. At a pH above the pKa the acid is deprotonated. If the pH equals the pKa, the acid is 50% protonated and 50% deprotonated. Attribution: Ivy Jose
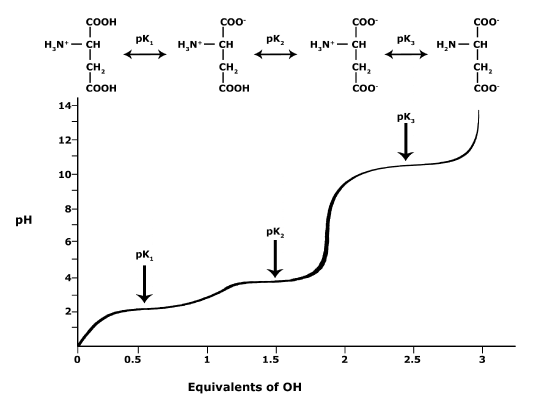
In BIS2A, we will be looking at the protonation state and deprotonation state of amino acids. Amino acids contain multiple functional groups that can be acids or bases. Therefore their protonation/deprotonation status can be more complicated. Below is the relationship between the pH and pKa of the amino acid Glutamic Acid. In this graph we can ask the question we posed earlier: Will the functional groups on the amino acid Glutamate be protonated or deprotonated at a pH of 2, at a pH of 8, at a pH of 11?
Figure 5. This graph depicts the protonation state of glutamate as the pH changes. At a pH below the pKa for each functional group on the amino acid, the functional group is protonated. At a pH above the pKa for the functional group it is deprotonated. If the pH equals the pKa, the functional group is 50% protonated and 50% deprotonated.
Attribution: Ivy Jose
Note: Possible discussion
- What is the overall charge of free Glutamate at a pH of 5?
- What is the overall charge of free Glutamate at a pH of 10?
Lipids
Lipids are a diverse group of hydrophobic compounds that include molecules like fats, oils, waxes, phospholipids, and steroids. Most lipids are at their core hydrocarbons, molecules that include many nonpolar carbon-carbon or carbon-hydrogen bonds. The abundance of nonpolar functional groups give lipids a degree of hydrophobic (“water fearing”) character and most lipids have low solubility in water. Depending on their physical properties (encoded by their chemical structure), lipids can serve many functions in biological systems including energy storage, insulation, barrier formation, cellular signaling. The diversity of lipid molecules and their range of biological activities are perhaps surprisingly large to most new students of biology. Let's start by developing a core understanding of this class of biomolecules.
Fats and oils
A common fat molecule or triglyceride. These types of molecules are generally hydrophobic and, while they have numerous functions, are probably best known for their roles in body fat and plant oils. A triglyceride molecule derived from two types of molecular components—a polar "head" group and a nonpolar "tail" group. The "head" group of a triglyceride is derived from a single glycerol molecule. Glycerol, a carbohydrate, is composed of three carbons, five hydrogens, and three hydroxyl (-OH) functional groups. The nonpolar fatty acid "tail" group consists of three hydrocarbons (a functional group composed of C-H bonds) that also have a polar carboxyl functional group (hence the term "fatty acid"—the carboxyl group is acidic at most biologically relevant pHs). The number of carbons in the fatty acid may range from 4–36; most common are those containing 12–18 carbons.
Note: possible discussion
The models of the triglycerides shown above depict the relative positions of the atoms in the molecule. If you Google for images of triglycerides you will find some models that show the phospholipid tails in different positions from those depicted above. Using your intuition, give an opinion for which model you think is a more correct representation of real life. Why?
Figure 2. Stearic acid is a common saturated fatty acid; oleic acid and linolenic acid are common unsaturated fatty acids. Attribution: Marc T. Facciotti (own work)
Note: possible discussion
Natural fats like butter, canola oil, etc., are composed mostly of triglycerides. The physical properties of these different fats vary depending on two factors:
- The number of carbons in the hydrocarbon chains;
- The number of desaturations, or double bonds, in the hydrocarbon chains.
The first factor influences how these molecules interact with each other and with water, while the second factor dramatically influences their shape. The introduction of a double bond causes a "kink" in the otherwise relatively "straight" hydrocarbon, depicted in a slightly exaggerated was in Figure 3.
Based on what you can understand from this brief description, propose a rationale—in your own words—to explain why butter is solid at room temperature while vegetable oil is liquid.
Here is an important piece of information that could help you with the quesion: butter has a greater percentage of longer and saturated hydrocarbons in its triglycerides than does vegetable oil.
Sterols
Steroids are lipids with a fused ring structure. Although they do not resemble the other lipids discussed here, they are designated as lipids because they are also largely composed of carbons and hydrogens, are hydrophobic, and are insoluble in water. All steroids have four linked carbon rings. Many steroids also have the -OH functional group which puts them in the alcohol classification of sterols. Several steroids, like cholesterol, have a short tail. Cholesterol is the most common steroid. It is mainly synthesized in the liver and is the precursor to many steroid hormones such as testosterone. It is also the precursor to Vitamin D and of bile salts which help in the emulsification of fats and their subsequent absorption by cells. Although cholesterol is often spoken of in negative terms, it is necessary for the proper functioning of many animal cells, particularly in its role as a component of the plasma membrane where it is known to modulate membrane structure, organization, and fluidity.
Note: possible discussion
In the molecule of cortisol above, what parts of the molecule would you classify as functional groups? Is there any disagreement over what should and should not be included as a functional group?
| Cholesterol | Cortisol |
Phospholipids
Phospholipids are major constituents of the cell membrane, the outermost layer of cells. Like fats, they are composed of fatty acid chains attached to glycerol molecule. Unlike the triacylglycerols, phospholipids have two fatty acid tails and a phosphate group attached to the sugar. Phospholipid are therefore amphipathic molecules, meaning it they have a hydrophobic part and a hydrophilic part. The two fatty acid chains extending from the glycerol are hydrophobic and cannot interact with water, whereas the phosphate-containing head group is hydrophilic and interacts with water. Can you identify the functional groups on the phospholipid below that give each part of the phospholipid its properties?
Note
Make sure to note in Figure 5 that the phosphate group has an R group linked to one of the oxygen atoms. R is a variable commonly used in these types of diagrams to indicate that some other atom or molecule is bound at that position. That part of the molecule can be different in different phospholipids—and will impart some different chemistry to the whole molecule. At the moment, however, you are responsible for being able to recognize this type of molecule (no matter what the R group is) because of the common core elements—the glycerol backbone, the phosphate group, and the two hydrocarbon tails.
| 1,2-Dioleoyl-sn-glycero-3-phospho-L-serine |
In the presence of water, some phospholipids will spontaneously arrange themselves into a micelle (Figure 6). The lipids will be arranged such that their polar groups will be on the outside of the micelle, and the nonpolar tails will be on the inside. Under other conditions, a lipid bilayer can also form. This structure, only a few nanometers thick, is composed of two opposing layers of phospholipids such that all the hydrophobic tails align face-to-face in the center of the bilayer and are surrounded by the hydrophilic head groups. A phospholipid bilayer forms as the basic structure of most cell membranes and are responsible for the dynamic nature of the plasma membrane.
Note: possible discussion
As mentioned above, if you were to take some pure phospholipids and drop them into water that some of the phospholipid would spontaneously form into micelles. This sounds like a process that could be described by an Energy Story.
Go back to the Energy Story rubric and try to create an Energy Story for this process — I expect that the steps involving the description of energy might be difficult at this point (we'll come back to that later) but you should be able to do at least the first three steps. You can also constructively critique each other's work to create an optimized story.
The phospholipid membrane is discussed in detail in a later module. It will be important to remember the chemical properties associated with the functional groups in the phospholipid in order to understand the function of the cell membrane.
Carbohydrates
Carbohydrates are one of the four main classes of macromolecules that make up all cells and are an essential part of our diet; grains, fruits, and vegetables are all natural sources. While we may be most familiar with the role carbohydrates play in nutrition, they also have a variety of other essential functions in humans, animals, plants, and bacteria. In this section, we will discuss and review basic concepts of carbohydrate structure and nomenclature, as well as a variety of functions they play in cells.
Molecular structures
In their simplest form, carbohydrates can be represented by the stoichiometric formula (CH2O)n, where n is the number of carbons in the molecule. For simple carbohydrates, the ratio of carbon-to-hydrogen-to-oxygen in the molecule is 1:2:1. This formula also explains the origin of the term “carbohydrate”: the components are carbon (“carbo”) and the components of water (“hydrate”). Simple carbohydrates are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides, which will be discussed below. While simple carbohydrates fall nicely into this 1:2:1 ratio, carbohydrates can also be structurally more complex. For example, many carbohydrates contain functional groups (remember them from our basic discussion about chemistry) besides the obvious hydroxyl. For example, carbohydrates can have phosphates or amino groups substituted at a variety of sites within the molecule. These functional groups can provide additional properties to the molecule and will alter its overall function. However, even with these types of substitutions, the basic overall structure of the carbohydrate is retained and easily identified.
Nomenclature
One issue with carbohydrate chemistry is the nomenclature. Here are a few quick and simple rules:
- Simple carbohydrates, such as glucose, lactose, or dextrose, end with an "-ose."
- Simple carbohydrates can be classified based on the number of carbon atoms in the molecule, as with triose (three carbons), pentose (five carbons), or hexose (six carbons).
- Simple carbohydrates can be classified based on the functional group found in the molecule, i.e ketose (contains a ketone) or aldose (contains an aldehyde).
- Polysaccharides are often organized by the number of sugar molecules in the chain, such as in a monosaccharide, disaccharide, or trisaccharide.
For a short video on carbohydrate classification, see the 10-minute Khan Academy video by clicking here.
Monosaccharides
Monosaccharides ("mono-" = one; "sacchar-" = sweet) are simple sugars; the most common is glucose. In monosaccharides, the number of carbons usually ranges from three to seven. If the sugar has an aldehyde group (the functional group with the structure R-CHO), it is known as an aldose; if it has a ketone group (the functional group with the structure RC(=O)R'), it is known as a ketose.

Figure 1. Monosaccharides are classified based on the position of their carbonyl group and the number of carbons in the backbone. Aldoses have a carbonyl group (indicated in green) at the end of the carbon chain and ketoses have a carbonyl group in the middle of the carbon chain. Trioses, pentoses, and hexoses have three, five, and six carbons in their backbones, respectively. Attribution: Marc T. Facciotti (own work)
Glucose versus galactose
Galactose (part of lactose, or milk sugar) and glucose (found in sucrose, glucose disaccharride) are other common monosaccharides. The chemical formula for glucose and galactose is C6H12O6; both are hexoses, but the arrangements of the hydrogens and hydroxyl groups are different at position C4. Because of this small difference, they differ structurally and chemically and are known as chemical isomers because of the different arrangement of functional groups around the asymmetric carbon; both of these monosaccharides have more than one asymmetric carbon (compare the structures in the figure below).
Fructose versus both glucose and galactose
A second comparison can be made when looking at glucose, galactose, and fructose (the second carbohydrate that with glucose makes up the disaccharide sucrose and is a common sugar found in fruit). All three are hexoses; however, there is a major structural difference between glucose and galactose versus fructose: the carbon that contains the carbonyl (C=O).
In glucose and galactose, the carbonyl group is on the C1 carbon, forming an aldehyde group. In fructose, the carbonyl group is on the C2 carbon, forming a ketone group. The former sugars are called aldoses based on the aldehyde group that is formed; the latter is designated as a ketose based on the ketone group. Again, this difference gives fructose different chemical and structural properties from those of the aldoses, glucose, and galactose, even though fructose, glucose, and galactose all have the same chemical composition: C6H12O6.
Figure 2. Glucose, galactose, and fructose are all hexoses. They are structural isomers, meaning they have the same chemical formula (C6H12O6) but a different arrangement of atoms.
Linear versus ring form of the monosaccharides
Monosaccharides can exist as a linear chain or as ring-shaped molecules. In aqueous solutions, monosaccharides are usually found in ring form (Figure 3). Glucose in a ring form can have two different arrangements of the hydroxyl group (OH) around the anomeric carbon (C1 that becomes asymmetric in the process of ring formation). If the hydroxyl group is below C1 in the sugar, it is said to be in the alpha (α) position, and if it is above C1 in the sugar, it is said to be in the beta (β) position.
Figure 3. Five- and six-carbon monosaccharides exist in equilibrium between linear and ring form. When the ring forms, the side chain it closes on is locked into an α or β position. Fructose and ribose also form rings, although they form five-membered rings as opposed to the six-membered ring of glucose.
Disaccharides
Disaccharides ("di-" = two) form when two monosaccharides undergo a dehydration reaction (also known as a condensation reaction or dehydration synthesis). During this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another monosaccharide, releasing a molecule of water and forming a covalent bond. A covalent bond formed between a carbohydrate molecule and another molecule (in this case, between two monosaccharides) is known as a glycosidic bond. Glycosidic bonds (also called glycosidic linkages) can be of the alpha or the beta type.
Figure 4. Sucrose is formed when a monomer of glucose and a monomer of fructose are joined in a dehydration reaction to form a glycosidic bond. In the process, a water molecule is lost. By convention, the carbon atoms in a monosaccharide are numbered from the terminal carbon closest to the carbonyl group. In sucrose, a glycosidic linkage is formed between the C1 carbon in glucose and the C2 carbon in fructose.
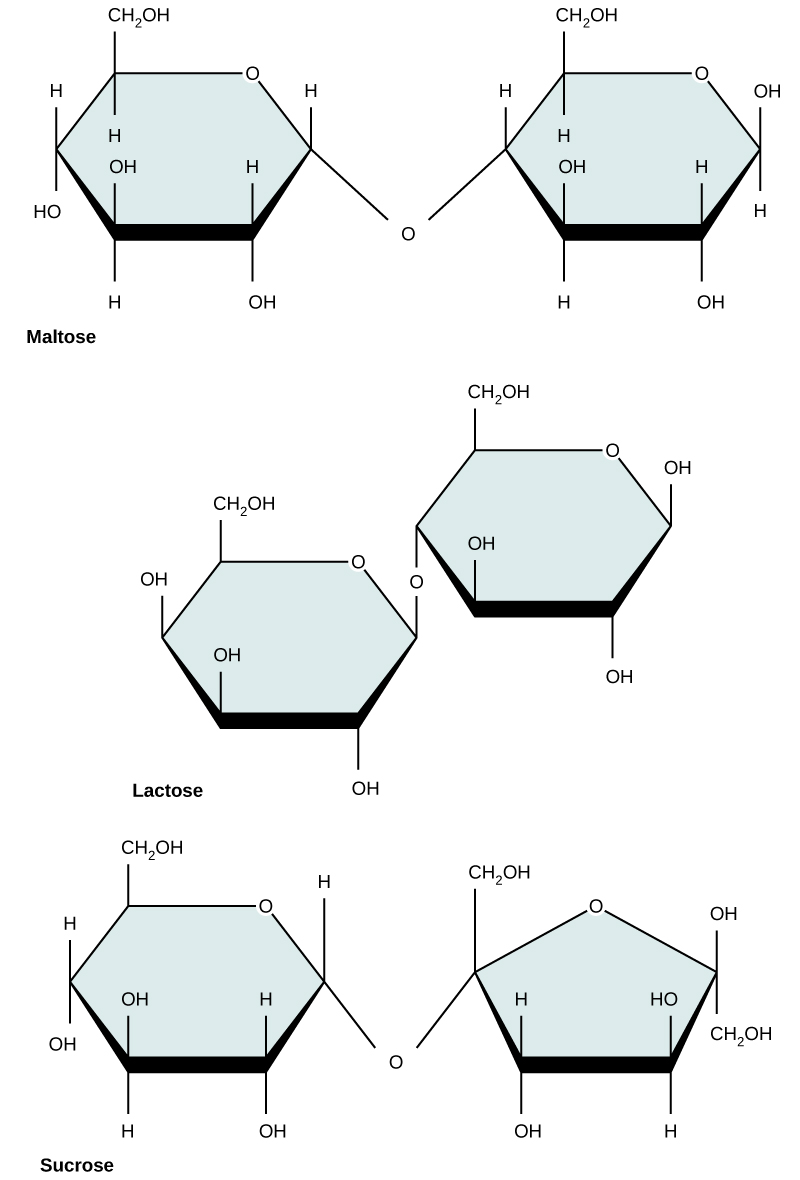
Common disaccharides include lactose, maltose, and sucrose (Figure 5). Lactose is a disaccharide consisting of the monomers glucose and galactose. It is found naturally in milk. Maltose, or malt/grain sugar, is a disaccharide formed by a dehydration reaction between two glucose molecules. The most common disaccharide is sucrose, or table sugar, which is composed of the monomers glucose and fructose.
Figure 5. Common disaccharides include maltose (grain sugar), lactose (milk sugar), and sucrose (table sugar).
| Sucrose | Lactose | Maltose |
Polysaccharides
A long chain of monosaccharides linked by glycosidic bonds is known as a polysaccharide ("poly-" = many). The chain may be branched or unbranched, and it may contain different types of monosaccharides. The molecular weight may be 100,000 Daltons or more, depending on the number of monomers joined. Starch, glycogen, cellulose, and chitin are primary examples of polysaccharides.
Starch is the stored form of sugars in plants and is made up of a mixture of amylose and amylopectin; both are polymers of glucose. Plants are able to synthesize glucose. Excess glucose, the amount synthesized that is beyond the plant’s immediate energy needs, is stored as starch in different plant parts, including roots and seeds. The starch in the seeds provides food for the embryo as it germinates and can also act as a source of food for humans and animals who may eat the seed. Starch that is consumed by humans is broken down by enzymes, such as salivary amylases, into smaller molecules, such as maltose and glucose.
Starch is made up of glucose monomers that are joined by 1-4 or 1-6 glycosidic bonds; the numbers 1-4 and 1-6 refer to the carbon number of the two residues that have joined to form the bond. As illustrated in Figure 6, amylose is starch formed by unbranched chains of glucose monomers (only 1-4 linkages), whereas amylopectin is a branched polysaccharide (1-6 linkages at the branch points).
Figure 6. Amylose and amylopectin are two different forms of starch. Amylose is composed of unbranched chains of glucose monomers connected by 1-4 glycosidic linkages. Amylopectin is composed of branched chains of glucose monomers connected by 1-4 and 1-6 glycosidic linkages. Because of the way the subunits are joined, the glucose chains have a helical structure. Glycogen (not shown) is similar in structure to amylopectin but more highly branched.
Glycogen
Glycogen is a common stored form of glucose in humans and other vertebrates. Glycogen is the animal equivalent of starch and is a highly branched molecule usually stored in liver and muscle cells. Whenever blood glucose levels decrease, glycogen is broken down to release glucose in a process known as glycogenolysis.
| Glycogen |
Cellulose
Cellulose is the most abundant natural biopolymer. The cell wall of plants is mostly made of cellulose, which provides structural support to the cell. Wood and paper are mostly cellulosic in nature. Cellulose is made up of glucose monomers that are linked by β 1-4 glycosidic bonds.
Figure 7. In cellulose, glucose monomers are linked in unbranched chains by β 1-4 glycosidic linkages. Because of the way the glucose subunits are joined, every glucose monomer is flipped relative to the next one, resulting in a linear, fibrous structure.
Note: possible discussion
Cellulose is not very soluble in water in its crystalline state; this can be approximated by the stacked cellulose fiber depiction above. Can you suggest a reason for why (based on the types of interactions) it might be so insoluble?
As shown in the figure above, every other glucose monomer in cellulose is flipped over, and the monomers are packed tightly as extended, long chains. This gives cellulose its rigidity and high tensile strength—which is so important to plant cells. While the β 1-4 linkage cannot be broken down by human digestive enzymes, herbivores such as cows, koalas, buffalos, and horses are able, with the help of the specialized flora in their stomach, to digest plant material that is rich in cellulose and use it as a food source. In these animals, certain species of bacteria and protists reside in the rumen (part of the digestive system of herbivores) and secrete the enzyme cellulase. The appendix of grazing animals also contains bacteria that digest cellulose, giving it an important role in the digestive systems of ruminants. Cellulases can break down cellulose into glucose monomers that can be used as an energy source by the animal. Termites are also able to break down cellulose because of the presence of other organisms in their bodies that secrete cellulases.
Interactions with carbohydrates
We have just discussed the various types and structures of carbohydrates found in biology. The next thing to address is how these compounds interact with other compounds. The answer to that is that it depends on the final structure of the carbohydrate. Because carbohydrates have many hydroxyl groups associated with the molecule, they are therefore excellent H-bond donors and acceptors. Monosaccharides can quickly and easily form H-bonds with water and are readily soluble. All of those H-bonds also make them quite "sticky". This is also true for many disaccharides and many short-chain polymers. Longer polymers may not be readily soluble.
Finally, the ability to form a variety of H-bonds allows polymers of carbohydrates or polysaccharides to form strong intramolecular and intermolocular bonds. In a polymer, because there are so many H-bonds, this can provide a lot of strength to the molecule or molecular complex, especially if the polymers interact. Just think of cellulose, a polymer of glucose, if you have any doubts.