2.8: Protein Localization
- Page ID
- 8615
In eukaryotic cells, where reactions and proteins are often sequestered into specialized membrane-bound compartments (organelles), a system needs to be in place for the targeted movement of specific proteins from where they are made in the cell to where they are used in the cell. In this section we will discuss two major modes of protein localization. We will begin with the components of the endomembrane system. This system involves co-translational translocation across membranes, and later delivery and processing through various organelles via vesicles and motor protein-mediated transport. It is employed for proteins that function within the compartments of the endomembrane system, for proteins embedded in the plasma membrane, and for secreted proteins.
We will also discuss another mode of protein targeting and translocation. This broad class of protein-targeting mechanisms occurs strictly post-translationally, and directs proteins to the nucleus, the mitochondria, the plastid, and the peroxisomes. It shares certain concepts- such as signal peptides- with the endomembrane system targeting mentioned above.
Discussion: You have learned about many types of proteins so far in this class. Name a few of these proteins and their location within the cell. For example, where would you expect to find glycolytic enzymes?
Design challenge
Eukaryotic cells contain membrane-bound organelles that effectively separate materials, processes, and reactions from one another and from the cytoplasm. This in itself poses a problem: How can the cell control the location of materials between these organelles? More specifically, how can a eukaryotic cell transport compounds from their place of origin (in most cases the cytoplasm) to where they are needed (perhaps the nucleus, the mitochondria, or the cell surface)?
Does the above design challenge look familiar? This challenge was introduced in the Eukaryotes unit of the course. We discussed the need for networking highways that allowed directional movement of vesicles from one location to another.
Protein Translocation and the Endomembrane System
In eukaryotes all mRNAs are bound by cytoplasmic ribosomes, and translation initiates in the cytoplasm. For some proteins, however, the first few (most N-terminal) amino acids exist not to provide protein function (they will be excised later anyway, as we will see) but rather to provide the protein's address. If the protein being translated needs to be transported to elsewhere (basically, anywhere but the cytoplasm) it will contain a signal sequence, a continuous stretch of amino acids that are recognized by a signal recognition particle (SRP). Various SRPs recognize various signal sequences.
If a protein is destined to become part of the endomembrane system (the ER, golgi, endosomes, plasma membrane, or secreted from the cell) the emergence of the signal peptide and its capture by its cognate SRP will cause the ribosome to stall. Translation will not be able to continue until the SRP has been removed. The resulting complex will diffuse through the cell until it comes into contact with an SRP receptor on the surface of the rough ER. The ribosome bound to the SRP will “dock” on the rough ER’s surface at a transmembrane channel called a translocon. When the ribosome binds to the translocon the SRP is released and the ribosome can continue translating the protein. The translocon will open wide enough for the newly created protein to feed directly into the lumen or the membrane of the rough ER.
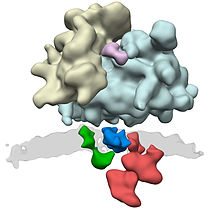
ER Translocon complex. Polymerization takes place in the ribosomes (yellow and light blue). Through the ER translocon (green: Sec61, blue: TRAP complex, and red: oligosaccharyl transferase complex), the newly synthesized protein is transported across the membrane (gray) into the interior of the ER. Sec61 is the protein-conducting channel and the OST adds sugar moieties to the nascent protein.
Discussion: When the SRP binds to the growing peptide chain and the ribosome, the ribosome stalls. What do you think is the significance of this? What would you predict would happen if the ribosome could continue translating the mRNA while the SRP was bound?
Endomembrane System
From this point the protein has entered into the endomembrane system. The rough ER’s membrane is the site of production for all transmembrane proteins as well as the proteins for the Golgi apparatus, lysosomes, endosomes, cell membrane and more. With the many options for protein destinations, the rough ER is involved in co-translational sorting of the proteins containing certain signal sequences. Proteins destined for similar locations will be packaged together in transport vesicles that bud off of the rough ER membrane. These vesicles fuse to form the cis flake of the golgi (closest to the nucleus). The lumen of golgi contains enzymes that recognize specific sequences on their target proteins, modifying their targets. Many types of post-translational modifications occur as the protein matures in the golgi:. Phosphorylation of oligosaccharides on lysosomal proteins occurs in the cis side of the Golgi apparatus, while galactose, sialic acid and other carbohydrates can be added to proteins in the trans side of the Golgi apparatus. These post-translational modifications can alter the protein’s structure and function and may also act as a signal that helps define the final destination of the protein. By the time the proteins emerge (again, in vesicles) different proteins with the same address have been sorted into the same vesicles (you might want to visualize this process here).
.svg.png?revision=1)
The endomembrane system
Vesicular movement (also described in the "Eukaryotes" module) is an energy intensive process that involves the use of microtubules and motor proteins. Vesicles are too large to be able to diffuse effectively within the cell. Motor proteins work to transport vesicles and their cargo by walking unidirectionally along microtubules. As the motor proteins move along the microtubule, chemical energy in the form of ATP is being converted into mechanical energy.
How do vesicles "know" they have reached their destination?
Microtubule based trafficking gives vesicles repeated pushes or pulls in a general direction (i.e., away from the nucleus). In cells that are much longer than they are wide (neurons, root hairs) vesicular trafficking can be quite dramatic. However, kinesins do not "know" that they are headed for, for example, the plasma membrane vs. the lysosomal membrane. They're just helping their cargo diffuse. In fact, it is the vesicle itself that recognizes its destination (though without the motor protein it would be unable to move). SNARE proteins embedded in the vesicle and its target recognize one another and facilitate the fusion of the vesicle with the membrane of its target compartment. For example, these proteins facilitate the release of neurotransmitters, allowing neurons to transmit intercellular signals. These same SNAREs are the target of many neurotoxins, include botulinum toxin (Botox). The inhibition of neurotransmitter release by Botox induces flaccid paralysis of muscles.
Energy story: Write an energy story for vesicular transport with in the cell. Include a description of the overall process and the initial and final states of the energy being used in the process. Overall, what work is being done?
Other Protein Transport Systems
There are many organelles that do not have their proteins delivered via the endomembrane system, such as the nucleus, the mitochondria and chloroplasts. Proteins sorted into non-endomembrane compartments do encode signal peptides, but their translocation is not coupled to translation. These proteins are completely translated in the cytoplasm, though they are often bound immediately by chaperonins that preclude their folding.
Protein Translocation into the Nucleus
Discussion: Make a hypothesis about how scientists might go about discovering a nuclear localization signal. What are some experiments/data that they would need in order to determine if a sequence was a nuclear localization signal?
Nuclear pore complexes are called “gated” channels that function as selective gates that can transport specific macromolecules into and out of the nucleus. Smaller proteins (<30 kDal) do not need to be recognized by this complex but can instead diffuse through freely. However, larger proteins need to carry a particular peptide motif termed a nuclear localization signals (NLS).
Nuclear localization signals are recognized by importins, small proteins that recognize and bind to the nuclear localization signal. A protein bound to importins is able to interact with the nuclear pore complex and pass through the channel into the nucleus. The importin acts as a "passport" to the nucleus.
Discussion: There are a few different nuclear localization signals found in nuclear proteins. These signals contain multiple lysine and arginine residues. What are the chemical characteristics of lysine and arginine? What does this tell you about the possible chemical characteristics of importins?
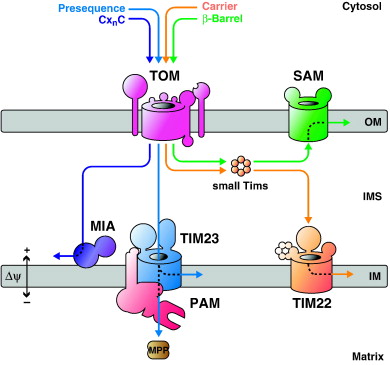
Protein translocation into Mitochondria
Roughly 10% of the proteins in a eukaryotic cell are in the mitochondria. The mitochondrion itself only synthesizes about 11-12 proteins, and therefore must import the majority of its proteins from the cytoplasm. Mitochondrial proteins are synthesized to completion before passing through the mitochondrial membrane. These proteins have an N-terminal targeting signal that folds into a strongly basic (+ charged) amphipathic alpha helix. Once this signal sequence emerges from the ribosome, a cytoplasmic chaperone protein will recognize the signal, bind to it and eventually dock the protein/ribosome complex to the mitochondrial outer membrane.
On the surface of the mitochondria, the N-terminus signal sequence is inserted into a protein-conducting channel (called Tom) which moves the protein through the outer membrane and -depending on the signal sequences present- into the inner membrane protein-conducting channel (called Tim). Note that there are many possible destinations for a mitochondrial protein (as there are for plastid proteins). Signal sequences within the protein itself will interact with recognition proteins at the mitochondrion to determine the protein's final location.
A mitochondrial proteins' final destination (outer membrane, intermembrane space, inner membrane, or matrix) will be determined by its particular array of signal sequences.