Electron Transport System
- Page ID
- 1327
Mitochondria
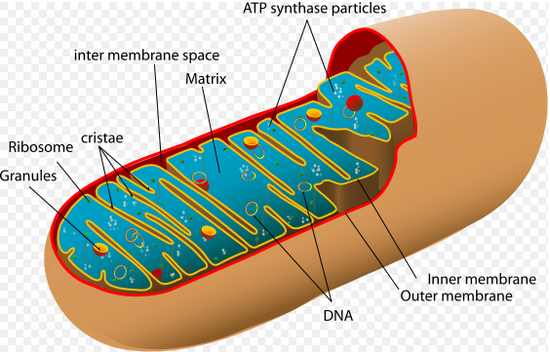
Mitochondria have two membranes, an inner membrane and an outer membrane. The inner membrane is much larger than the outer membrane, but is connected to inner foldings called cristae. In between the inner and outer membrane there is an intermembrane space and the space inside of the inner membrane is called the matrix, which contains enzymes and other reagents. The outer membrane is permeable to other molecules, because it contains a mitochondrial porin, which is a pore-forming protein. The inner membrane has an impermeable membrane to most ions and polar molecules and only permeable to ATP, pyruvate and citrate because of metabolite transporters.
Electron Transport System
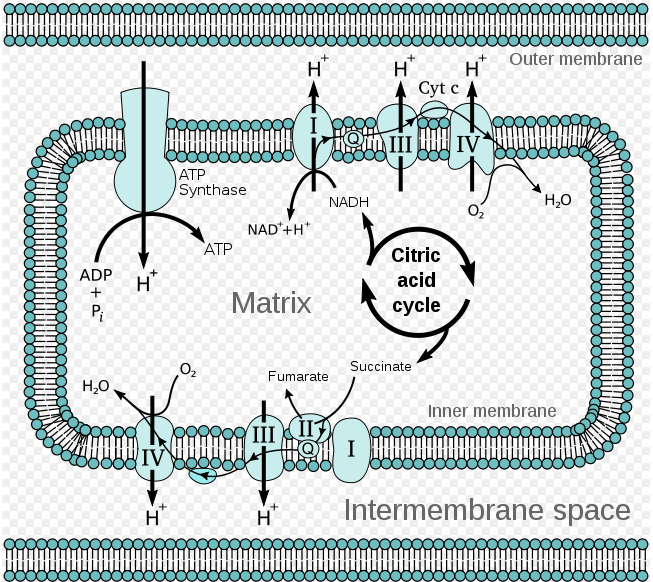
As shown from this diagram, electron flow from NADH to O2 is facilitated by several intermediate electron carriers, for example electrons move from a reduced donor, such as malate, to an oxidized donor, such as OAA. The electrons move from carrier to carrier, down a favorable energy gradient to the final electron acceptor, O2. The favorable energy gradient is due to the fact that electrons move towards components with a more positive reduction potential and thus a higher affinity for oxygen. As the electron flow occurs, protons are flowing across the inner membrane into the intermembrane space.
Components of the Electron Transport Chain
When electrons flow down the energy gradient from NADH to O2, the four protein complexes that catalyze the redox reaction are:
- Complex I = NADH-Q reductase complex
- Complex III= Cytochrome c reductase complex
- Cyt C = Cytochrome c
- Complex IV = Cytochrome c oxidase complex
When electrons flow down the energy gradient from FADH2 to O2, the four protein complexes that catalyze the redox reaction are:
- Complex II = succinate dehydrogenase
- Complex III= Cytochrome c reductase complex
- Cyt C = Cytochrome c
- Complex IV = Cytochrome c oxidase complex
Note: Electrons from FADH2 enter the electron transport chain at the fourth protein complex, succinate-Q reductase. This protein complex contains succinate dehydrogenase which was responsible for generating FADH2 from the reaction converting succinate into fumarate. Since FADH2 electrons enter the electron transport chain later in the cycle, they pump less H+ ions to the intermembrane space (protein complex II does not pump H+ ions), which means less ATP is generated.
Coenzyme Q:
Why do electrons flow in this direction?
We determine electron flow by calculating energy relationships from e- affinity and redox voltages
Change in Eo’ : “standard” reduction potential @ 1 M reduced form (donor), 1 M oxidized form (acceptor), 25 degrees C, pH 7 (Like the phosphoryl- transfer potential is measured by ΔG, the electron transfer potential is measured by ΔEo)
The measurement of redox potential: We can measure ΔEo’ relative to 1M H+/ saturated H2 gas We can measure the electron-transfer potential of an electron by setting up the following experiment. There are two electrical terminals, one contains the sample cell solution containing 1M reduced form (1M NADH) and 1M oxidized form (1M NAD+) and the other contains the standard reference cell, 1M H+ and 1M saturated H2 gas. The electrodes in the two solutions are connected by voltmeter. The voltmeter measures the voltage between the two terminals. Electrons flow from one cell to the other. If electrons flow from the sample cell to the standard reference cell and have a negative voltage, We would have an electrode in solution connected to another electrode in standard reference cell. By measuring the voltage between terminals, .
Relationship between the standard free energy change standard delta G and the reduction potential standard delta E:

Standard reduction potentials for some reactions:
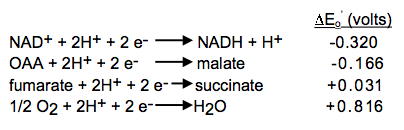
Combine the reactions and standard reduction potentials: Reverse the donor half-reaction and multiply its reduction potential by -1; add the reactions and reduction potentials
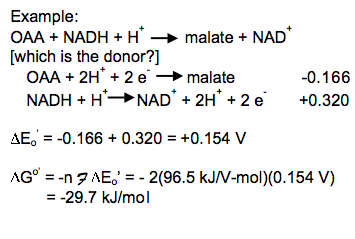
Combine the reactions and standard reduction potentials: Reverse the donor half-reaction and multiply its reduction potential by -1; add the reactions and reduction potentials
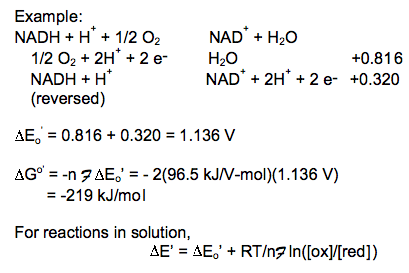
In this reaction a significant amount of energy is released by the reduction of O2 with NADH. The free energy released in this reaction generates the proton gradient that drives the synthesis of ATP and transport of other metabolites.

