2.1: Carbohydrates- structure and diversity in biology
- Page ID
- 3647
Carbohydrate Review for BIS103
1. Definition: Carbohydrates (CHOs=sugars=saccharides): Polyhydroxyaldehydes or polyhydroxyketones or substances which give these on hydrolysis. Most (not all) have the empirical formula of [CH2O]n. I abbreviate carbohydrates as CHOs in this wiki.
2. Fischer projection: We use two ways to visualize three dimensional CHOs on a two dimensional piece of paper. The first is the Fischer projection. The second is the Haworth projection (see below). For the Fischer projection, the molecule is arranged with the most oxidized end at the top and the carbon chain vertically oriented, with horizontal lines represent bonds projecting out of the plane of the paper toward us, and vertical lines represent bonds projecting behind the paper with OH groups arranged to the left or right. (devised by Hermann Emil Fischer in 1891).
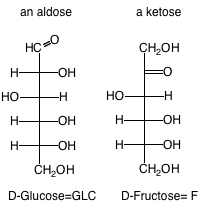
3. Aldose or Ketose:Refers to whether a saccharide in its open form contains an aldehyde or ketone carbonyl, respectively.
4. Monosaccharides: Carbohydrates that cannot be hydrolyzed to simpler compounds. As they are introduced in class, learn the structures of the following aldose monosaccharides: glyceraldehyde, erythrose, ribose, glucose, mannose, galactose; and the following ketose monosaccharides: dihydroxyacetone, ribulose, xylulose, fructose.
5. Triose, Tetrose, Pentose, etc.: Refers to the number of carbons in the monosaccharide. Often combined names such as aldohexose, etc. are used.
6. Disaccharides, Oligosaccharides, Polysaccharides, etc.: Refers to the number of monosaccharide units the carbohydrate can be hydrolyzed into. Check out what hydrolysis means here: http://chemwiki.ucdavis.edu/Core/Organic_Chemistry/Glossary/Hydrolysis
7. Steroisomers, Enantiomers, Diastereomers, Meso Compounds: Stereoisomers are compounds having the same molecular formula and same structure, but different orientations of their atoms in space. In other words, stereoisomers have the same types of bonds, but different spatial arrangements and different chemical properties.
Biological systems can tell the difference between stereoisomers!
Carbohydrates have one or more chiral centers- carbons with 4 different groups attached, giving rise to the possibility of stereoisomers Generally, a compound containing “n” chiral centers can maximally have 2“n” stereoisomers. Enantiomers are stereoisomeric compounds whose structures are nonsuperimposible mirror images of each other. Diastereomers are stereoisomeric compounds that are not mirror images of each other. Epimers are diastereomers that differ in configuration at only one chiral carbon.
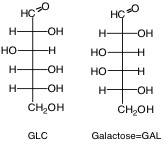
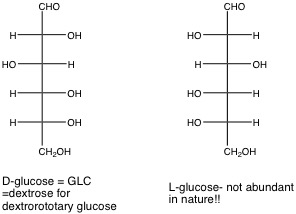
8. D- or L- Structure: Refers to whether the configuration at the chiral carbon furthest from the carbonyl group for a monosaccharide (for example, highest-numbered stereocenter is C5 of GLC or Fructose) in its open form in the Fischer projection resembles that for R-(+) or L-S-(-)-glyceraldehyde (2,3-dihydroxypropanal), respectively. The D forms of sugars predominate in nature, so we will leave off the D- or L- designation. It is assumed, unless written otherwise, that all sugars presented in this class are D sugars. For example, in the Fischer projection, the D form of GLC has the C-5 hydroxyl projecting to the right.
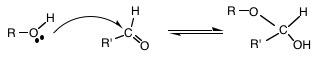
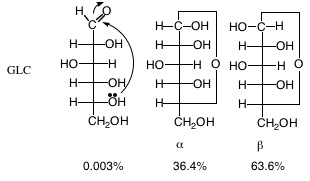
9. Furanose, Pyranose: Carbohydrates with five or more carbons cyclize to form 5-membered furanose or 6-membered pyranose ring structures. The result of the addition of the aldehyde carbonyl to an alcohol results in a hemiacetal; addition of the keto carbonyl to an alcohol results in a hemiketal. Because hemiacetal and hemiketal reactions can occur with intramolecular groups, a cyclic structure is formed with an oxygen as a member of the ring. This intramolecular reaction occurs at a significant rate without enzymes. Combined names such as glucopyranose, etc. are often used. Cyclization creates a new chiral carbon (see #10 below).
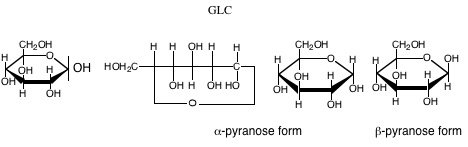
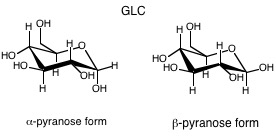
10. Haworth projection: Fischer representations are not as useful to visualize the ring form of CHOs. We use the Haworth projection to visualize the ring form, as a planar ring with OH groups above and below the ring. Does not represent true shape of rings, they are puckered. For pyranose rings, the two conformations are the chair and the boat. Substituents are either equatorial or axial.
If drawn to the left in a Fischer projection, the constituent is above the ring in the corresponding Haworth projection (and when to the right in Fischer, below in Haworth).
But, remember that the structures look more like this:
11. \(\alpha\)- or \(\beta\)- Anomers: Refers to the configuration at the anomeric (=previously carbonyl) carbon in the cyclic form of a monosaccharide or monosaccharide derivative. Specifically in aldohexoses, it is related to whether the substituent is on the opposite (\(\alpha\)) or same (\(\beta\)) side of the molecule as the –CH2OH group (C6). Anomers are a subcategory of epimers in which the chiral center in question is the anomeric carbon.
12. Mutarotation: A process in which a solution of a carbohydrate undergoes a change in optical rotation through conversion of one anomer via the open-chain form into an equilibrium mixture of both anomers. On a molecular basis, the chemical process which occurs is called anomerization.
13. Reducing or Non-Reducing Sugars: Refers to whether a carbohydrate gives a positive or a negative test, respectively, \(\mathrm{CU^{2+}}\) or \(\mathrm{Ag^{+}}\) species in Benedict’s and Fehling’s or Tollen’s Reagents. All reducing sugars contain a free or potentially free aldehyde moiety (as a hemiacetal or hemiketal) that can reduce \(\mathrm{Cu^{2+}}\) to \(\mathrm{Cu^{+}}\). An alkaline \(\mathrm{CuSO_{4}}\)solution is Fehling’s solution. A reducing sugar produces a red cuprous oxide (\(\mathrm{Cu_{2}O}\)) precipitate.
14. Glycosides: A general term referring to monosaccharide derivatives in which the functional group involving the anomeric carbon has an acetal or ketal structure. Condensation can occur with alcohols, amines, and amides. Glycoside formation locks the anomeric carbon such that it can not undergo mutarotation.

