4.4: Exceptions to autosomal inheritance
- Page ID
- 25736
Learning Objectives
- Compare different modes of sex determination, such as mammalian XY, fruit flies, insects, or environmental.
- Explain why dosage compensation is needed and the role of the non-coding RNA Xist in mammalian dosage compensation.
- Predict the number of Barr bodies in a particular cell.
- Predict offspring phenotypes for X-linked genes.
For loci on autosomes, in which all individuals have two copies of the chromosome and either copy is equally likely to be passed to offspring, the alleles follow the normal Mendelian pattern of inheritance. However, for loci on the allosomes (also called sex chromosomes) individuals may have zero, one, or two copies of a chromosome. Because homologous chromosomes contain the same genes, individuals may have zero, one, or two copies of some genes. In humans, the allosomes are the X and Y chromosomes, which contain different genes, even though they act as a homologous pair during meiosis. Instead, genes on the X chromosome will follow an X-linked pattern of inheritance, while genes on the Y chromosome will follow a Y-linked pattern of inheritance.
X-Linked inheritance example: the white gene in Drosophila melanogaster
A well-studied sex-linked gene is the white gene on the X chromosome of Drosophila melanogaster. Normally flies have red eyes but flies with a mutant allele of this gene called white- (w-) have white eyes because the red pigments are absent. Because this mutation is recessive to the wild type w+ allele females that are heterozygous have normal red eyes. Female flies that are homozygous for the mutant allele have white eyes. Because there is no white gene on the Y chromosome, male flies can only be hemizygous for the wild type allele or the mutant allele.
Note about nomenclature
In genetics, genes are often named after their mutant (frequently, but not always, loss-of-function) phenotypes. Remember, genetics has its origin in observing phenotypes. The white allele described here was identified in 1910 (Morgan, T.H.) long before gene sequences could be determined, in fact, even before DNA had been identified as the unit of heredity. The DNA sequence of this locus was not published until 1984 (O'Hare et al). We know now that this gene encodes a transporter protein that deposits pigment and that the mutant allele does not produce a functional transporter, but the gene name white continues to be used.
The process of identifying mutant phenotypes and then later determining which gene is involved is called forward genetics.
The alternative, to identify a candidate gene and manipulate it to observe the phenotype, is called reverse genetics, and was only made possible by the advent of modern molecular biology tools.
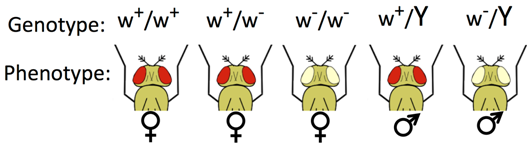
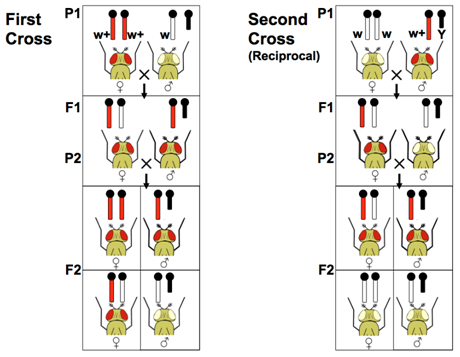
A researcher may not know beforehand whether a novel mutation is sex-linked. The definitive method to test for sex-linkage is reciprocal crosses, in which a male and a female that have different phenotypes are crossed. In a second cross, the phenotypes are reversed relative to the sex of the parents in the first cross. Whenever reciprocal crosses give different results in the F1 and F2, and whenever the male and female offspring have different phenotypes, the usual explanation is sex-linkage. Remember, if the locus were autosomal, the F1 and F2 progeny are expected to show the same genotypes and phenotypes from either of these crosses.
A similar pattern of sex-linked inheritance is seen for X-chromosome loci in other species with an XX-XY sex chromosome system, including mammals and humans. The ZZ-ZW system is similar, but reversed (see below).
Diversity in Sex Determination
Sex determination refers to the process by which reproductive organs are specified to become ovaries or testes. Mechanisms for sex determination in plants and animals are remarkably diverse, and include sex chromosomes, chromosome dosage, and environment among others.
Some organisms used chromosomal sex determination. For example in humans and other mammals XY embryos commonly develop testes while XX embryos develop ovaries. This difference in development is usually initiated by the presence of a single gene, the SRY gene (sex-determining region on Y), on the Y-chromosome. SRY encodes is a transcription factor that activates expression of genes that promote testes differentiation. Without SRY, these genes are not activated and genes that promote ovary development are transcribed. Notably, SRY initiates the process of testes development, but a series of genes are required to complete testes and reproductive organ development. If the downstream genes are not fully functional, development of these structures may not occur despite the presence of the Y chromosome and SRY.
Query \(\PageIndex{1}\)
Although Drosophila melanogaster also has a Y chromosome, there is not an SRY gene and the X to Autosome (X:A) ratio determines male or female differentiation. Individuals with two autosome sets and two X chromosomes (2A:2X) will develop ovaries, while those with only one X chromosome (2A:1X) will develop testes. The presence/absence of the Y-chromosome and its genes are not significant. For example, both an XO and XY fruit fly will develop testes and other male phenotypes, if they both have two sets of autosomes.
In other animal species, the number of chromosome sets can determine sex. For example the haploid-diploid system is used in bees, ants, and wasps. Typically haploid individuals, which develop from unfertilized eggs, develop testes, while diploid individuals, which develop from fertilized eggs, form ovaries. For this system to work, meiosis does not occur during sperm production in haploids; instead sperm are identical copies produced by mitosis because chromosome number does not need to be reduced by half.
In other species, the environment can determine an individuals sex. In alligators (and some other reptiles) the temperature of development dictates the sex, while in many reef fish, the population sex ratio can produce environmental signals that cause some individuals to change sex.
Dosage Compensation
Mammals and Drosophila both have XX - XY sex determination systems. However, because these systems evolved independently they work differently with regard to compensating for the difference in gene dosage. Remember, in most cases the sex chromosomes act as a homologous pair even though the Y chromosome has lost most of the loci when compared to the X chromosome. Comparison of X and Y chromosomes had led to the view that Y chromosomes have evolved from autosomes that degenerated, slowly mutating and losing loci. In modern day mammals, the Y chromosomes have very few genes, while the X chromosomes remain as they were. This is a general feature of all organisms that use chromosome based sex determination systems. Chromosomes found in both sexes (the X or the Z) have retained their genes while the chromosome found in only one sex (the Y or the W) have lost most of their genes.
Human X and Y Compared
| X chromosome | Y chromosome | |
| Number of base pairs | 156,040,895 | 57,227,415 |
| Number of coding genes | 857 | 64 |
| Contains essential genes for viability? | Yes | No |
Ensembl 2021. (Release 104) Nucleic Acids Res. 2021, vol. 49(1):884–891 PubMed PMID: 33137190. doi:10.1093/nar/gkaa942
Chromosomal sex determination requires dealing with the gene dosage difference between individuals with different chromosome complements: e.g. XX females have two copies ("doses") of X-chromosome genes while XY males only have one copy of these genes. For all the autosomes, XX and XY individuals have the same number of copies of genes. Producing too much of some proteins can be problematic, even lethal, for organisms. This difference in gene dosage needs to be compensated in a process called dosage compensation. In Drosophila and many other insects, individuals with single X chromosome express the genes on it at twice the normal rate. This mechanism of dosage compensation restores a balance of proteins encoded by X-linked genes and those made by autosomal genes.
X-chromosome Inactivation in Mammals
In mammals the dosage compensation system operates in any cell with more than one X chromosome. In XX embryos, one X in each cell is randomly chosen and marked for inactivation by expressing a long non-coding RNA Xist (X inactive specific transcript). The Xist RNA stays in the nucleus and "coats" the X chromosome, recruiting proteins that affect chromosome packing. This X chromosome becomes highly compact heterochromatin. The expression of Xist is repressed on the other chromosome, so it remains more loosely packed euchromatin. The Xi is replicated during S phase and transmitted during mitosis the same as any other chromosome, but most of its genes are never allowed to turn on and the chemical modifications that regulate chromatin are passed on following each round of cell division.
Make a prediction
What would happen to XY or XX mouse embryos that lack the Xist gene?
- Answer
-
XY mouse embryos with a deletion of the Xist gene were viable and fully fertile. The XX mouse embryos died during embryonic development.
(Marahrens Y, Panning B, Dausman J, Strauss W, Jaenisch R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 1997 Jan 15;11(2):156-66. doi: 10.1101/gad.11.2.156. PMID: 9009199.)
From this point forward this chromosome will be inactive, abbreviated Xinactive (Xi). The chromosome appears as a condensed mass within interphase nuclei called the Barr body. With the inactivation of genes on one X chromosome, XX individuals have the same number of functioning X-linked genes as XY individuals. The other X chromosome, the Xactive (Xa), is unaffected. The mechanisms that determine X inactivation can also be used in other situations, such as XXX, in which cells inactivate two X chromosomes, or XXY individuals, in which cells inactivate one X chromosome. This remarkable system always leaves one X chromosome active, such that every mammalian cell expresses the correct dose of X chromosome genes.
Counting chromosomes
The exact mechanism cells use to "count" their X chromosomes is not entirely clear. Essentially all mammalian cells leave only one X chromosome active, even when abnormal numbers of X chromosomes are present.
Examples:
- Females with an extra X (XXX genotype) have 2 Barr bodies and 1 active X.
- Males with an extra X (XXY) have 1 Barr body and 1 active X.
This random inactivation of one X-chromosome leads to a commonly observe phenomenon in cats. A familiar X-linked gene is the Orange gene (O) in cats. The OO allele encodes an enzyme that results in orange pigment for the hair. The OB allele causes the hairs to be black. Heterozygous females have an orange and black mottled phenotype known as tortoiseshell. This is due to patches of skin cells having different X-chromosomes inactivated. In each orange hair the Xi chromosome carrying the OB allele is inactivated. The OO allele on the Xa is functional and orange pigments are made. In black hairs the reverse is true, the Xi chromosome with the OO allele is inactive and the Xa chromosome with the OB allele is active. Because the inactivation decision happens early during embryogenesis, the cells continue to divide to make large patches on the adult cat skin where one or the other X is inactivated. The Orange gene in cats is a good demonstration of how the mammalian dosage compensation system affects gene expression. However, most X-linked genes do not produce such dramatic mosaic phenotypes in heterozygous females.
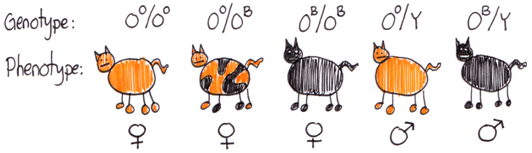
OB allele = black. (Original-Harringtion-CC:AN)
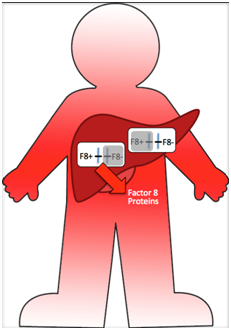
Another example is the F8 gene in humans that encodes Factor VIII, a protein needed for blood clotting that is produced in liver cells. If an XY individual is hemizygous for a mutant allele (F8–/F8–) the result is hemophilia type A. An XX individual homozygous for mutant alleles will also have hemophilia. Heterozygous F8+/F8– individuals do not have hemophilia because even though half of their liver cells do not make Factor VIII (because the X with the F8+ allele is inactive) the other 50% can. Because some of their liver cells are exporting Factor VIII proteins into the blood stream, they have the ability to form blood clots throughout their bodies. The genetic mosaicism in the cells of their bodies does not produce a visible mosaic phenotype.
Example \(\PageIndex{1}\)
Predicting genetic crosses with X-linked genes follows that of autosomal genes, remembering that each gamete contains only one sex chromosome, unless otherwise specified.
A tortoiseshell female cat and a black male cat have 8 offspring. How many are expected to be tortoiseshell?
Solution
Using a Punnett square reveals that 25% (or two) offspring are predicted to be tortoiseshell.
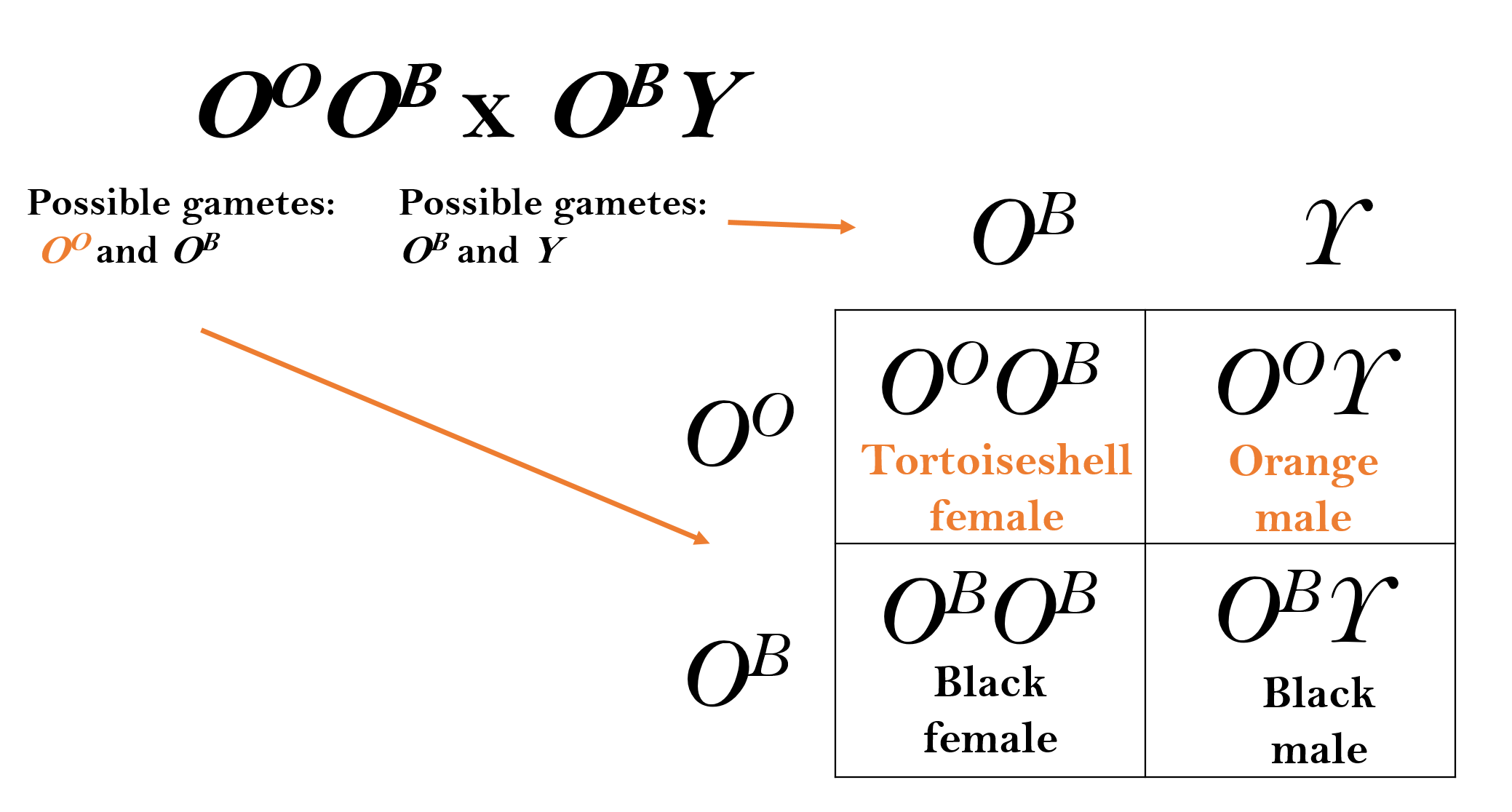
Exercise \(\PageIndex{1}\)
If you were able to clone a tortoiseshell cat, would the pattern of orange and black markings be identical in the clone? Why or why not?
- Answer
-
The pattern is not likely to be identical because X chromosome inactivation happens during embryogenesis. When the cloned cat is in the early stages of development, the X chromosomes with the orange and black alleles will be randomly inactivated and passed on during each mitosis as more cells are formed.
Exercise \(\PageIndex{2}\)
A heterozygous individual (genotype F8+/F8—) is a carrier for the hemophilia allele. Her partner is an XY male with normal blood clotting. What is the probability their son will have hemophilia? What about a daughter?
- Answer
-
A son will be either F8+Y or F8—Y (50% with hemophilia). A daughter will be either F8+/F8— or F8+/F8+ (0% with hemophilia).
Z-linked genes
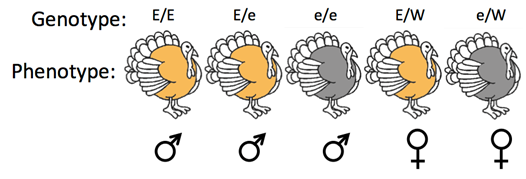
The ZW system is distinguished from the XY system based on which sex has two different sex chromosomes, also called the heterogametic sex. In this case, male birds commonly have ZZ chromosomes, whereas female birds commonly have ZW chromosomes. Thus, female birds may have only one copy of Z-linked genes. For example, a Z-linked gene influences feather color in turkeys. Turkeys are birds, which use the ZZ-ZW sex chromosome system. The E allele makes the feathers bronze and the e allele makes the feathers brown (Figure \(\PageIndex{5}\)). Only turkeys with two Z chromosomes, commonly males, can be heterozygous for this locus. Heterozygous turkeys (Ee) are uniformly bronze because the E allele is completely dominant to the e allele and birds use a dosage compensation system similar to Drosophila and not mammals. Reciprocal crosses between turkeys from pure-breeding bronze and brown breeds would reveal that this gene is in fact Z-linked.
Contributors and Attributions
Dr. Todd Nickle and Isabelle Barrette-Ng (Mount Royal University) The content on this page is licensed under CC SA 3.0 licensing guidelines.
References
Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman T-L, et al. (2014) Sex Determination: Why So Many Ways of Doing It? PLoS Biol 12(7): e1001899. https://doi.org/10.1371/journal.pbio.1001899


