21.2: Carbohydrates
- Page ID
- 23731
skills to develop
- Give examples of monosaccharides and polysaccharides
- Describe the function of monosaccharides and polysaccharides within a cell
The most abundant biomolecules on earth are carbohydrates. From a chemical viewpoint, carbohydrates are primarily a combination of carbon and water, and many of them have the empirical formula (CH2O)n, where n is the number of repeated units. This view represents these molecules simply as “hydrated” carbon atom chains in which water molecules attach to each carbon atom, leading to the term “carbohydrates.” Although all carbohydrates contain carbon, hydrogen, and oxygen, there are some that also contain nitrogen, phosphorus, and/or sulfur. Carbohydrates have myriad different functions. They are abundant in terrestrial ecosystems, many forms of which we use as food sources. These molecules are also vital parts of macromolecular structures that store and transmit genetic information (i.e., DNA and RNA). They are the basis of biological polymers that impart strength to various structural components of organisms (e.g., cellulose and chitin), and they are the primary source of energy storage in the form of starch and glycogen.
Monosaccharides: The Sweet Ones
In biochemistry, carbohydrates are often called saccharides, from the Greek sakcharon, meaning sugar, although not all the saccharides are sweet. The simplest carbohydrates are called monosaccharides, or simple sugars. They are the building blocks (monomers) for the synthesis of polymers or complex carbohydrates, as will be discussed further in this section. Monosaccharides are classified based on the number of carbons in the molecule. General categories are identified using a prefix that indicates the number of carbons and the suffix –ose, which indicates a saccharide; for example, triose (three carbons), tetrose (four carbons), pentose (five carbons), and hexose (six carbons) (Figure \(\PageIndex{1}\)). The hexose D-glucose is the most abundant monosaccharide in nature. Other very common and abundant hexose monosaccharides are galactose, used to make the disaccharide milk sugar lactose, and the fruit sugar fructose.
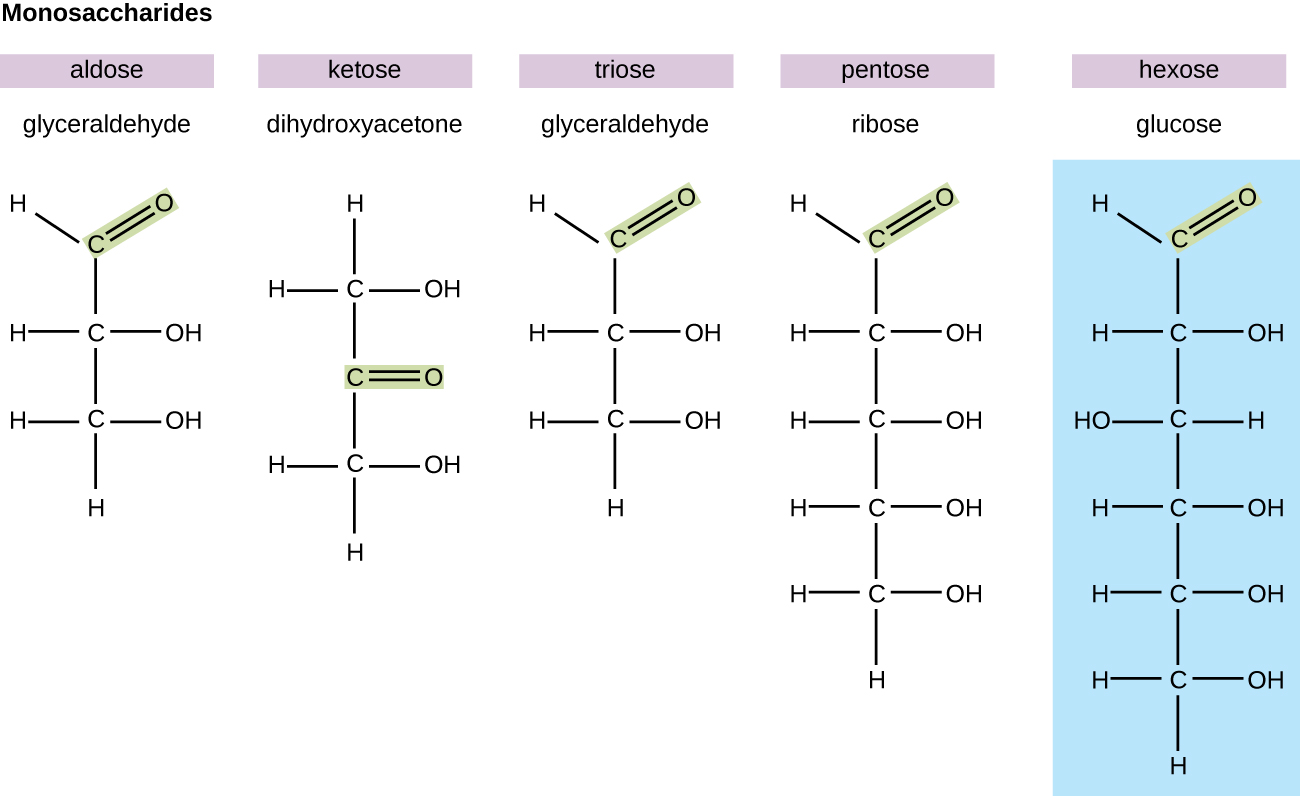
Figure \(\PageIndex{1}\): Monosaccharides are classified based on the position of the carbonyl group and the number of carbons in the backbone.
Monosaccharides of four or more carbon atoms are typically more stable when they adopt cyclic, or ring, structures. These ring structures result from a chemical reaction between functional groups on opposite ends of the sugar’s flexible carbon chain, namely the carbonyl group and a relatively distant hydroxyl group. Glucose, for example, forms a six-membered ring (Figure \(\PageIndex{2}\)).
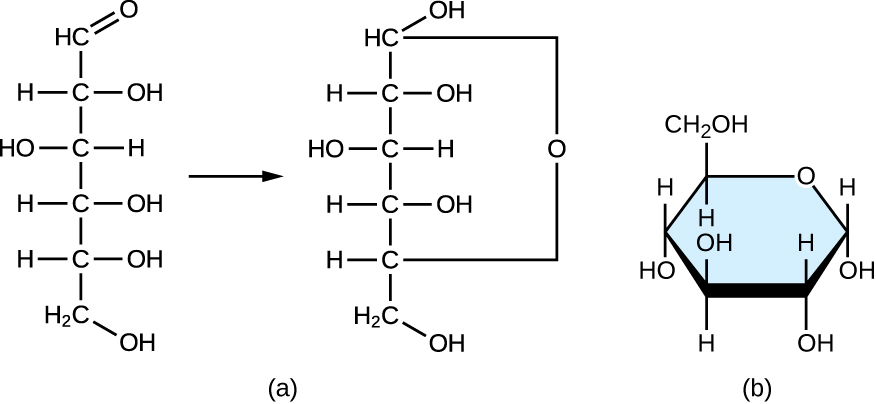
Figure \(\PageIndex{2}\): (a) A linear monosaccharide (glucose in this case) forms a cyclic structure. (b) This illustration shows a more realistic depiction of the cyclic monosaccharide structure. Note in these cyclic structural diagrams, the carbon atoms composing the ring are not explicitly shown.
Exercise \(\PageIndex{1}\)
Why do monosaccharides form ring structures?
Disaccharides
Two monosaccharide molecules may chemically bond to form a disaccharide. The name given to the covalent bond between the two monosaccharides is a glycosidic bond. Glycosidic bonds form between hydroxyl groups of the two saccharide molecules, an example of the dehydration synthesis described in the previous section of this chapter:
monosaccharide—OH+HO—monosaccharide⟶monosaccharide—O—monosaccharide⨽⨼−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−disaccharidemonosaccharide—OH+HO—monosaccharide⟶monosaccharide—O—monosaccharide⎵disaccharide
Common disaccharides are the grain sugar maltose, made of two glucose molecules; the milk sugar lactose, made of a galactose and a glucose molecule; and the table sugar sucrose, made of a glucose and a fructose molecule (Figure \(\PageIndex{3}\)).
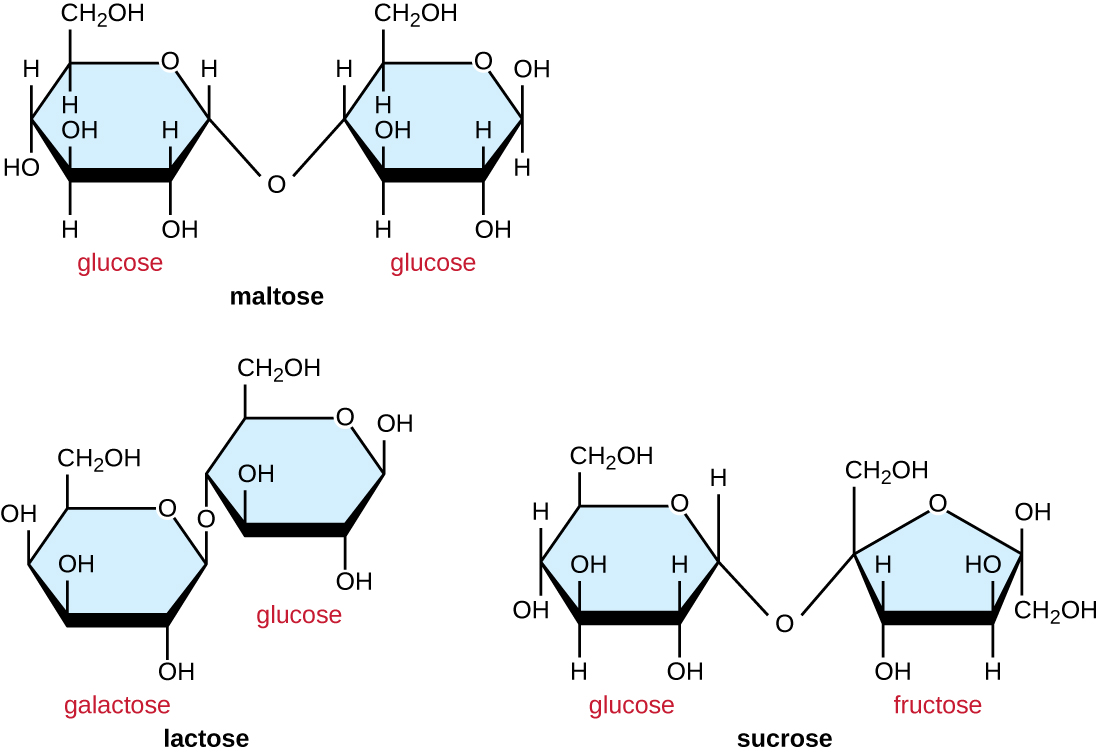
Figure \(\PageIndex{3}\): Common disaccharides include maltose, lactose, and sucrose.
Polysaccharides
Polysaccharides, also called glycans, are large polymers composed of hundreds of monosaccharide monomers. Unlike mono- and disaccharides, polysaccharides are not sweet and, in general, they are not soluble in water. Like disaccharides, the monomeric units of polysaccharides are linked together by glycosidic bonds.
Polysaccharides are very diverse in their structure. Three of the most biologically important polysaccharides—starch, glycogen, and cellulose—are all composed of repetitive glucose units, although they differ in their structure (Figure \(\PageIndex{4}\)). Cellulose consists of a linear chain of glucose molecules and is a common structural component of cell walls in plants and other organisms. Glycogen and starch are branched polymers; glycogen is the primary energy-storage molecule in animals and bacteria, whereas plants primarily store energy in starch. The orientation of the glycosidic linkages in these three polymers is different as well and, as a consequence, linear and branched macromolecules have different properties.
Modified glucose molecules can be fundamental components of other structural polysaccharides. Examples of these types of structural polysaccharides are N-acetyl glucosamine (NAG) and N-acetyl muramic acid (NAM) found in bacterial cell wall peptidoglycan. Polymers of NAG form chitin, which is found in fungal cell walls and in the exoskeleton of insects.
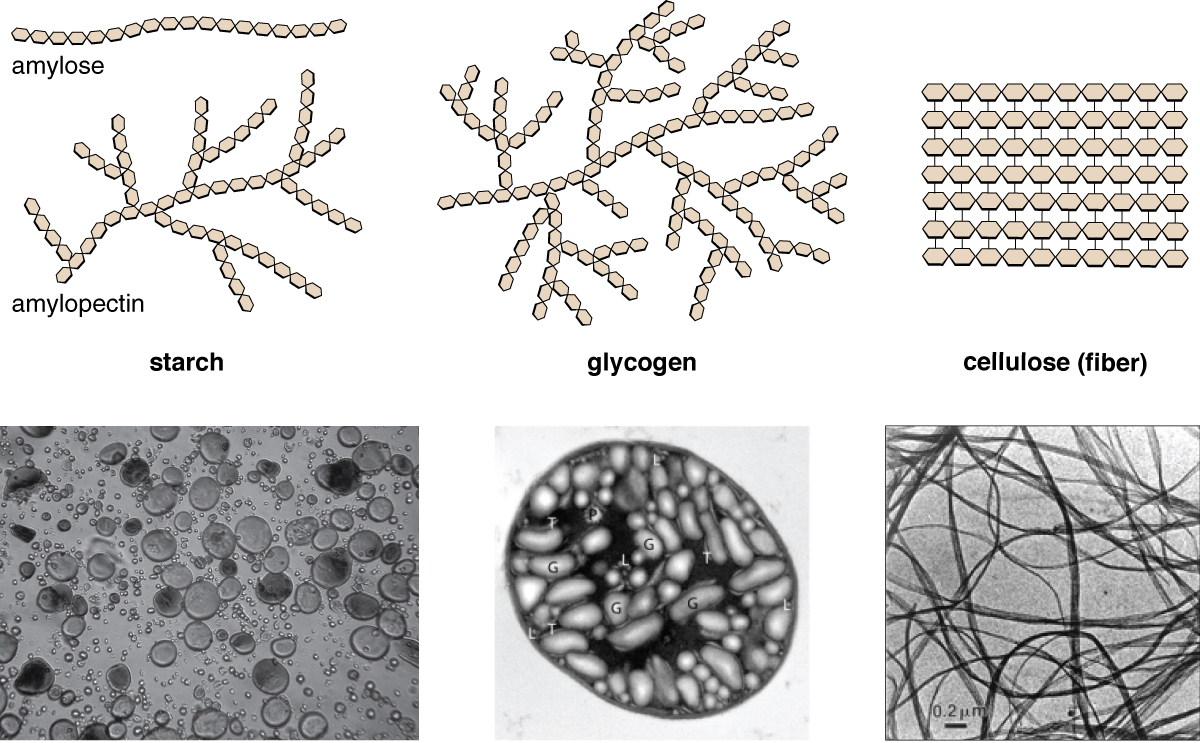
Figure \(\PageIndex{4}\): Starch, glycogen, and cellulose are three of the most important polysaccharides. In the top row, hexagons represent individual glucose molecules. Micrographs (bottom row) show wheat starch granules stained with iodine (left), glycogen granules (G) inside the cell of a cyanobacterium (middle), and bacterial cellulose fibers (right). (credit “iodine granules”: modification of work by Kiselov Yuri; credit “glycogen granules”: modification of work by Stöckel J, Elvitigala TR, Liberton M, Pakrasi HB; credit “cellulose”: modification of work by American Society for Microbiology)
Exercise \(\PageIndex{2}\)
What are the most biologically important polysaccharides and why are they important?
Key Concepts and Summary
- Carbohydrates, the most abundant biomolecules on earth, are widely used by organisms for structural and energy-storage purposes.
- Carbohydrates include individual sugar molecules (monosaccharides) as well as two or more molecules chemically linked by glycosidic bonds. Monosaccharides are classified based on the number of carbons the molecule as trioses (3 C), tetroses (4 C), pentoses (5 C), and hexoses (6 C). They are the building blocks for the synthesis of polymers or complex carbohydrates.
- Disaccharides such as sucrose, lactose, and maltose are molecules composed of two monosaccharides linked together by a glycosidic bond.
- Polysaccharides, or glycans, are polymers composed of hundreds of monosaccharide monomers linked together by glycosidic bonds. The energy-storage polymers starch and glycogen are examples of polysaccharides and are all composed of branched chains of glucose molecules.
- The polysaccharide cellulose is a common structural component of the cell walls of organisms. Other structural polysaccharides, such as N-acetyl glucosamine (NAG) and N-acetyl muramic acid (NAM), incorporate modified glucose molecules and are used in the construction of peptidoglycan or chitin.
Multiple Choice
By definition, carbohydrates contain which elements?
A. carbon and hydrogen
B. carbon, hydrogen, and nitrogen
C. carbon, hydrogen, and oxygen
D. carbon and oxygen
C
Monosaccharides may link together to form polysaccharides by forming which type of bond?
A. hydrogen
B. peptide
C. ionic
D. glycosidic
D
Matching
Match each polysaccharide with its description.
| ___chitin | A. energy storage polymer in plants |
| ___glycogen | B. structural polymer found in plants |
| ___starch | C. structural polymer found in cell walls of fungi and exoskeletons of some animals |
| ___cellulose | D. energy storage polymer found in animal cells and bacteria |
C, D, A, B
Short Answer
What are monosaccharides, disaccharides, and polysaccharides?
Critical Thinking
The figure depicts the structural formulas of glucose, galactose, and fructose. (a) Circle the functional groups that classify the sugars either an aldose or a ketose, and identify each sugar as one or the other. (b) The chemical formula of these compounds is the same, although the structural formula is different. What are such compounds called?

Structural diagrams for the linear and cyclic forms of a monosaccharide are shown. (a) What is the molecular formula for this monosaccharide? (Count the C, H and O atoms in each to confirm that these two molecules have the same formula, and report this formula.) (b) Identify which hydroxyl group in the linear structure undergoes the ring-forming reaction with the carbonyl group.

The term “dextrose” is commonly used in medical settings when referring to the biologically relevant isomer of the monosaccharide glucose. Explain the logic of this alternative name.
Contributor
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


