6.5: Lab Procedures- Testing Oxygen requirements
- Page ID
- 52333
Learning Outcomes
- Inoculating thioglycollate tubes and TSA plates for incubation
Procedure
Thioglycollate broth
- The thioglycollate broth should be either boiled first before inoculation OR recently made so that the oxygen content is very low. (Your instructor will tell you if it needs to be boiled).
- Inoculate a tube of thioglycollate broth with your unknown bacterium: make sure that the loop or needle goes down to the BOTTOM of the broth (do not get metal holder in the sterile broth).
- Incubate at 25 or 37 degrees C as directed.
Watch Video 1: Deep stab agar slant inoculation
Watch video 1: This procedure is showing you how to inoculate a deep stab agar slant. A similar procedure can be used for inoculating thioglycollate tubes, except that the media is semi-solid agar and there is typically no slant, so you don't have to do that last zig-zagging streak on the top of the slant. (3:10) Video by Sinclair College eCourse Design & Development. URL: https://youtu.be/p4awvOVGZXA
TSA plates in 3 different oxygen environments
- Label 3 plates for the table---candle jar, ambient air, and GasPak anaerobic jar.
- Divide the 3 plates into sections, one for each organism—your unknown, the strict aerobe, the strict anaerobe.
- Inoculate the section by streaking a straight line or a zigzag (as seen below). HOWEVER, be sure that you inoculate all 3 plates using the same technique.
- Be sure that the jar has a methylene blue indicator strip (seen above) inside. The methylene blue is blue when oxidized, but colorless when reduced. Before the jar is opened, the strip should be checked to make sure that it is COLORLESS.
- Incubate at 30 or 37 degrees C
INTERPRETATION: after incubation
TSA plates
Compare the presence/absence of growth, as well as the quantity of growth on the 3 plates. Determine whether aerobic, anaerobic, or facultatively anaerobic.
To the right:
- A is an facultative anaerobe
- B is an aerobe (microaerophilic)
- C is an anaerobe
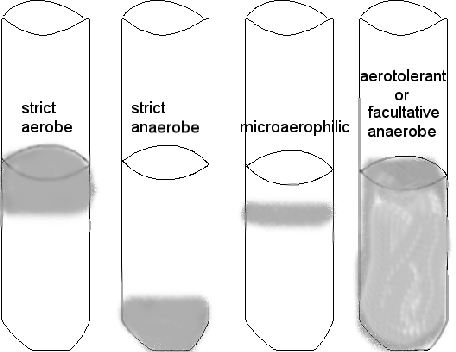
Thioglycollate broth
Determine WHERE the most amount of growth occurs in the column of liquid---the top, the bottom, top to bottom. DO NOT SHAKE IT! Can you determine if the bacterium is aerobic, anaerobic, or facultatively anaerobic?
Contributors
1. Jackie Reynolds, Professor of Biology (Richland College)


