2.1: Introduction Growth Media
- Page ID
- 52224
Learning Outcomes
- Recognize various types of growth media: solid, broth.
- Understand the uses of selective and differential growth media
- Determine the properties of some common bacterial types when grown on selective and differential growth media
Growth Media
To study bacteria and other microorganisms, it is necessary to grow them in controlled conditions in the laboratory. Growth media contain a variety of nutrients necessary to sustain the growth of microorganisms. There are two commonly used physical forms of growth media: liquid media and solid growth media. A liquid medium is called a broth (image 2).
Solid growth media usually contains agar (image 1), which is a mixture of polysaccharides derived from red algae. It is used as a solidification agent because it (1) is not broken down by bacteria, (2) contains no nutrients that can be used by bacteria and (3) melts at high temperatures, and yet is solid at temperatures used for most bacterial growth. Solid growth media is used in the following forms: agar plates, agar slants, and agar deeps. To make agar deeps or agar slants, melted agar is poured into a test tube and then allowed to solidify vertically (agar deep), or at a slant (agar slant). Agar plates are made by pouring melted agar into a petri dish.
Image 1: Solid Agar slant Image 2: Broth media in test tube. Images by Anne Hanson, University of Maine, Orono.
Image 3: MacConkey solid agar plate with pure culture of Salmonealla species bacteria. Image by Rebecca Buxton, University of Utah, Salt Lake City, UT.
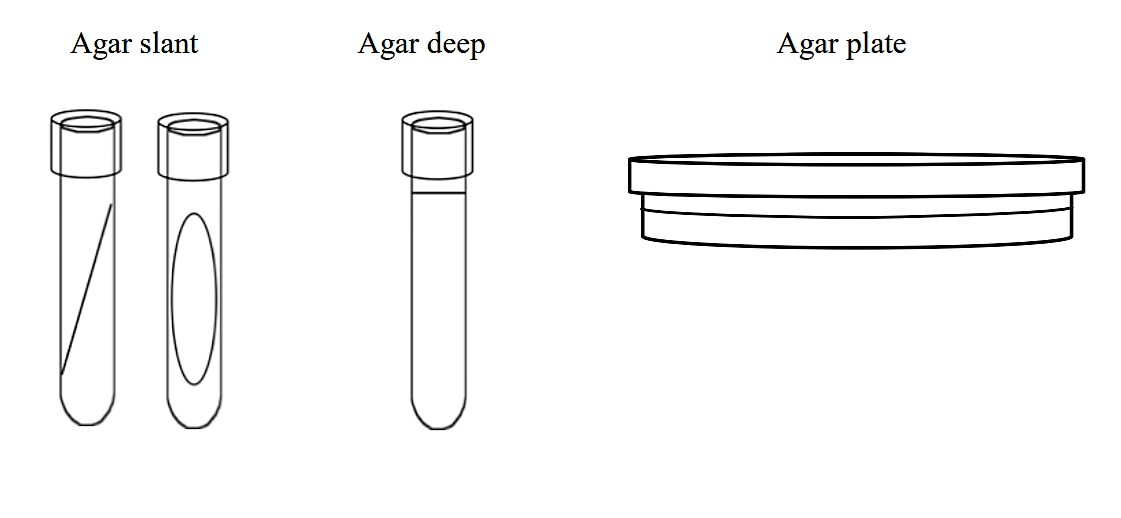.png?revision=1&size=bestfit&width=757&height=350) Figure 1: Solid growth media forms
Figure 1: Solid growth media forms
Broths can be used to determine growth patterns in a liquid medium, and for certain types of inoculations and metabolic tests. They are also the method of choice for growing large quantities of bacteria. Agar slants are commonly used to generate stocks of bacteria. Agar plates can be used to separate mixtures of bacteria and to observe colony characteristics of different species of bacteria . Deeps are used for several different types of differential metabolic tests (e.g., the caseinase hydrolysis test)
Types of Growth Media
Growth media can be categorized based on their chemical constituents, or the purpose for which they are used.
- Complex (also called Rich) growth media contain ingredients whose exact chemical composition is unknown (e.g. blood, yeast extract, etc.) Eg TSA, LB agar.
- Synthetic (also called chemically defined) growth media are formulated to an exactly defined chemical composition.
- Example: A general purpose growth medium: e.g. tryptic soy agar (TSA) or Luria broth (LB) is used to grow a wide variety of non-fastidious bacteria. This type of medium is often a complex growth medium.
Image 4: General purpose, Complex/Rich media: LB agar plate streaked with Bacillus cereus and incubated at room temperature for 24 hours. Image by Kevin Hedetniemi and Min-Ken Liao, Furman University, Greenville, SC.
Specialized media types
- A selective growth medium contains chemicals that allow some types of bacteria to grow, while inhibiting the growth of other types. An example of a purely selective growth medium is PEA, phenylethyl alcohol agar, which allows Gram positive bacteria to grow while inhibiting the growth of Gram negative bacteria.
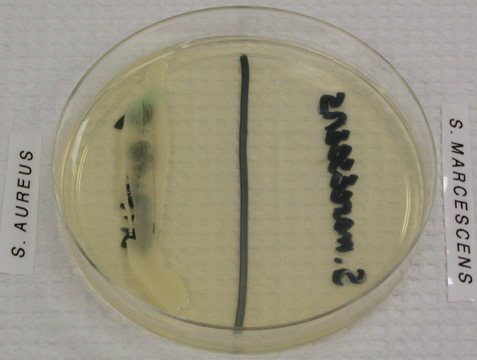
Image 5: Staphylococcus aureus, a Gram positive organism, grows on this PEA plate while Serratia marcescens, a Gram negative organism, does not. Image by WelcometoMicrobugz. URL: https://www.austincc.edu/microbugz/p...cohol_agar.php
- A differential growth medium is formulated such that different types of bacteria will grow with different characteristics (e.g. colony color). An example of a differential growth medium is blood agar, which differentiates among bacteria based on their ability to break down red blood cells and hemoglobin. Blood agar is also a complex growth medium because it contains blood.
Image 6: Blood agar plate. Image by Rebecca Buxton, University of Utah, Salt Lake City, UT.
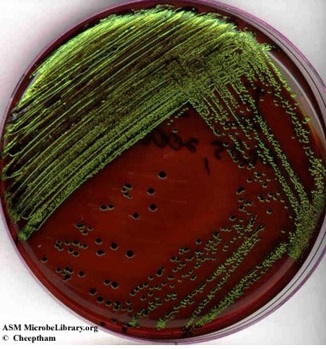
- A growth medium can be both selective and differential. For example, EMB (eosin methylene blue agar) inhibits the growth of Gram-positive bacteria. Gram negative bacteria that grow on this medium are differentiated based on their ability to ferment the sugars lactose and sucrose. Strong fermenters of lactose or sucrose will produce large amounts of acid and will appear dark purple to black. Escherichia coli, a strong fermenter will frequently appear as colonies with dark black with a green metallic sheen (Image 7). Weaker fermenters will produce pink colonies. Clear or colorless colonies indicate that bacterium does not ferment either sugar and is not a fecal coliform. (Note: the Gram staining procedure divides bacteria into 2 main groups: Gram-positive bacteria and Gram-negative bacteria, based on their cell wall structure.).
Image 7: Eosin-methylene blue ( EMB) agar plate inoculated with Escherichia coli (a Gram-negative coliform bacterium) showing good growth of dark blue-black colonies with metallic green sheen indicating vigorous fermentation of lactose and acid production which precipitates the green metallic pigment. (Naowarat Cheeptham, Thompson Rivers University, Kamloops, BC, Canada)
Making Media
I. Making culture media requires patience and attention to detail. Watch Video 1: Making Microbiological Media
Watch Video 1: Making Microbiological Media by Bio-Rad. URL:https://youtu.be/BH4ESgWU_Eo
II. Pouring Agar plates. Watch Video 2: Solid Media Preparation, video was filmed at NC State Microbiology labs.
Watch Video 1: Solid media preparation, video was filmed at NC State Microbiology labs. URL:https://youtu.be/P7M_MCXbZjc
Recap: Media Types
Tryptic soy agar (TSA): General purpose rich/complex growth medium.
Mannitol-salt agar (MSA): Differential and selective growth medium. This medium contains 7.5% NaCl, the carbohydrate mannitol and the pH indicator phenol red (yellow at pH 8.4). It is selective for staphylococci due to the high concentration of NaCl, and differentiates based on the ability to ferment mannitol. Staphylococci that ferment mannitol produce acidic byproducts that cause the phenol red to turn yellow. This produces a yellow halo in the medium around the bacterial growth.
| Selectivity | Interpretation | Identification |
|---|---|---|
| Growth | Growth Organism not inhibited by NaCl | E.g., Staphylococcus, Micrococcus |
| No Growth | Organism inhibited by NaCl | Not Staphylococcus |
| Differentiation | ||
| Yellow Halo | Organism ferments mannitol | Probable S. aureus |
| No Yellow Halo | Organism does not ferment mannitol | Staphylococcus species (other than S. aureus); Micrococcus (yellow colonies) |
Eosin-methylene blue agar (EMB): Differential and selective growth medium. This medium contains peptone, lactose, sucrose and the dyes eosin Y and methylene blue. Gram positive organisms are inhibited by the dyes, so this medium is selective for Gram negative bacteria. The medium differentiates based on the ability to ferment lactose (and/or sucrose.) Organisms that cannot ferment either of the sugars produce colorless colonies. Organisms that ferment the sugars with some acid production produce pink or purple colonies; organisms that ferment the sugars and produce large amounts of acid form colonies with a green metallic sheen. This medium is commonly used to detect the presence of fecal coliforms (like E. coli)—bacteria that grow in the intestines of warm-blooded animals. Fecal coliforms produce large amounts of acid when fermenting lactose and/or sucrose; non-fecal coliforms will produce less acid and appear as pink or purple colonies.
| Result | Identification | Interpretation |
|---|---|---|
| No or poor growth | Organism inhibited by dyes | Organism is Gram-positive |
| Good growth | Organism not inhibited by dyes | Organism is Gram-negative |
| Colorless growth | Organism does not ferment sucrose or lactose | Non-coliform |
| Growth is pink and mucoid | Organism ferments lactose and/or sucrose with some acid production | Coliform bacteria |
| Growth is dark (purple to black with or without green metallic sheen) | Organism ferments lactose and/or sucrose, with large amounts of acid production | Possible fecal coliform (E. coli) |
References
1) Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)