8.4: Other Environmental Conditions that Affect Growth
- Page ID
- 75855
- Identify and describe different categories of microbes with specific growth requirements such as altered barometric pressure, osmotic pressure, humidity, and light
- Describe how osmotic pressure functions and how it is affected by the different balances of solutes and water in and around the cell
- Identify and describe different types of transport across the cell membrane
Microorganisms interact with their environment along more dimensions than pH, temperature, and free oxygen levels, although these factors require significant adaptations. We also find microorganisms adapted to varying levels of barometric pressure, humidity, light, and salinity.
Barometric Pressure
Microorganisms that require high atmospheric pressure for growth are called barophiles. The bacteria that live at the bottom of the ocean must be able to withstand great pressures. Because it is difficult to retrieve intact specimens and reproduce such growth conditions in the laboratory, the characteristics of these microorganisms are largely unknown.
Light
Photoautotrophs, such as cyanobacteria or green sulfur bacteria, and photoheterotrophs, such as purple nonsulfur bacteria, depend on sufficient light intensity at the wavelengths absorbed by their pigments to grow and multiply. Energy from light is captured by pigments and converted into chemical energy that drives carbon fixation and other metabolic processes. The portion of the electromagnetic spectrum that is absorbed by these organisms is defined as photosynthetically active radiation (PAR). It lies within the visible light spectrum ranging from 400 to 700 nanometers (nm) and extends in the near infrared for some photosynthetic bacteria. A number of accessory pigments, such as fucoxanthin in brown algae and phycobilins in cyanobacteria, widen the useful range of wavelengths for photosynthesis and compensate for the low light levels available at greater depths of water. Other microorganisms, such as the archaea of the class Halobacteria, use light energy to drive their proton and sodium pumps. The light is absorbed by a pigment protein complex called bacteriorhodopsin, which is similar to the eye pigment rhodopsin. Photosynthetic bacteria are present not only in aquatic environments but also in soil and in symbiosis with fungi in lichens. The peculiar watermelon snow is caused by a microalga Chlamydomonas nivalis, a green alga rich in a secondary red carotenoid pigment (astaxanthin) which gives the pink hue to the snow where the alga grows.
- Which photosynthetic pigments were described in this section?
- What is the fundamental stress of a hypersaline environment for a cell?
Osmotic Pressure
Most natural environments tend to have lower solute concentrations than the cytoplasm of most microorganisms. Rigid cell walls protect the cells from bursting in a dilute environment. Not much protection is available against high osmotic pressure. In this case, water, following its concentration gradient, flows out of the cell. This results in plasmolysis (the shrinking of the protoplasm away from the intact cell wall) and cell death. This fact explains why brines and layering meat and fish in salt are time-honored methods of preserving food. Microorganisms called halophiles (“salt loving”) actually require high salt concentrations for growth. These organisms are found in marine environments where salt concentrations hover at 3.5%. Extreme halophilic microorganisms, such as the red alga Dunaliella salina and the archaeal species Halobacterium in Figure \(\PageIndex{1}\), grow in hypersaline lakes such as the Great Salt Lake, which is 3.5–8 times saltier than the ocean, and the Dead Sea, which is 10 times saltier than the ocean.

Dunaliella spp. counters the tremendous osmotic pressure of the environment with a high cytoplasmic concentration of glycerol and by actively pumping out salt ions. Halobacterium spp. accumulates large concentrations of K+ and other ions in its cytoplasm. Its proteins are designed for high salt concentrations and lose activity at salt concentrations below 1–2 M. Although most halotolerant organisms, for example Halomonas spp. in salt marshes, do not need high concentrations of salt for growth, they will survive and divide in the presence of high salt. Not surprisingly, the staphylococci, micrococci, and corynebacteria that colonize our skin tolerate salt in their environment. Halotolerant pathogens are an important cause of food-borne illnesses because they survive and multiply in salty food. For example, the halotolerant bacteria S. aureus, Bacillus cereus, and V. cholerae produce dangerous enterotoxins and are major causes of food poisoning.
Microorganisms depend on available water to grow. Available moisture is measured as water activity (aw), which is the ratio of the vapor pressure of the medium of interest to the vapor pressure of pure distilled water; therefore, the aw of water is equal to 1.0. Bacteria require high aw (0.97–0.99), whereas fungi can tolerate drier environments; for example, the range of aw for growth of Aspergillus spp. is 0.8–0.75. Decreasing the water content of foods by drying, as in jerky, or through freeze-drying or by increasing osmotic pressure, as in brine and jams, are common methods of preventing spoilage.
Osmotic Pressure and the Cellular Envelope
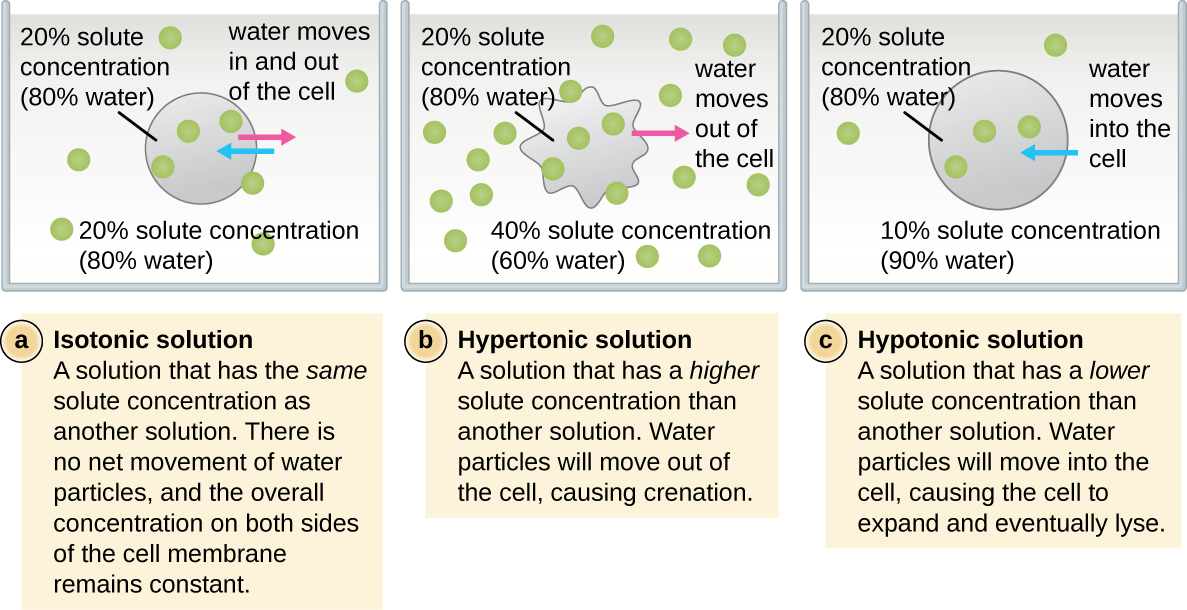
The cell wall is a structure found in most prokaryotes and some eukaryotes; it envelopes the cell membrane, protecting the cell from changes in osmotic pressure, i.e. water activity (Figure \(\PageIndex{2}\)). Osmotic pressure occurs because of differences in the concentration of solutes on opposing sides of a selectively or semipermeable membrane. Water is able to pass through a selectively or semipermeable membrane, but solutes (dissolved molecules like salts, sugars, and other compounds) cannot always. When the concentration of solutes is greater on one side of the membrane, water diffuses across the membrane from the side with the lower concentration of solutes (more water) to the side with the higher concentration of solutes (less water) until the concentrations on both sides become equal. This diffusion of water is called osmosis, and it can cause extreme osmotic pressure on a cell when its external environment changes.
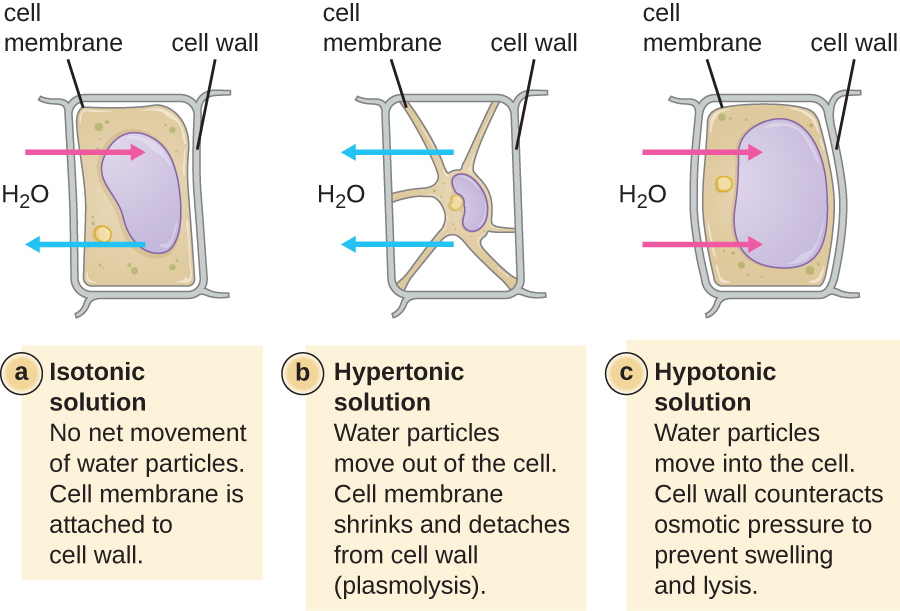
The external environment of a cell can be described as an isotonic, hypertonic, or hypotonic medium. In an isotonic medium, the solute concentrations inside and outside the cell are approximately equal, so there is no net movement of water across the cell membrane. In a hypertonic medium, the solute concentration outside the cell exceeds that inside the cell, so water diffuses out of the cell and into the external medium. In a hypotonic medium, the solute concentration inside the cell exceeds that outside of the cell, so water will move by osmosis into the cell. This causes the cell to swell and potentially lyse, or burst.
The degree to which a particular cell is able to withstand changes in osmotic pressure is called tonicity. Cells that have a cell wall are better able to withstand subtle changes in osmotic pressure and maintain their shape. In hypertonic environments, cells that lack a cell wall can become dehydrated, causing crenation, or shriveling of the cell; the plasma membrane contracts and appears scalloped or notched (Figure \(\PageIndex{2}\)). By contrast, cells that possess a cell wall undergo plasmolysis rather than crenation. In plasmolysis, the plasma membrane contracts and detaches from the cell wall, and there is a decrease in interior volume, but the cell wall remains intact, thus allowing the cell to maintain some shape and integrity for a period of time (Figure \(\PageIndex{3}\)). Likewise, cells that lack a cell wall are more prone to lysis in hypotonic environments. The presence of a cell wall allows the cell to maintain its shape and integrity for a longer time before lysing (Figure \(\PageIndex{3}\)).
Membrane Transport Mechanisms
One of the most important functions of the plasma membrane is to control the transport of molecules into and out of the cell. Internal conditions must be maintained within a certain range despite any changes in the external environment. The transport of substances across the plasma membrane allows cells to do so.
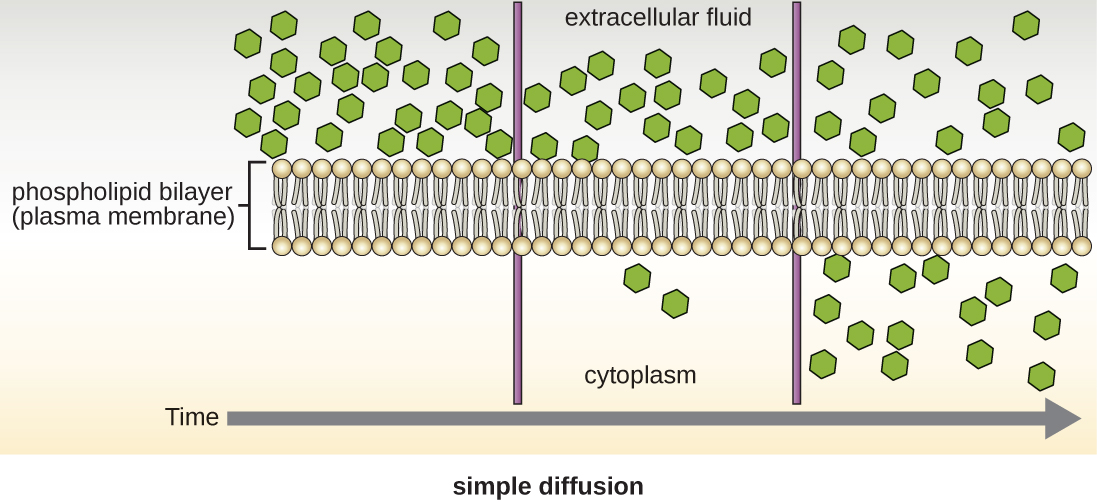
Passive Diffusion
Cells use various modes of transport across the plasma membrane. Collectively this ability to move molecules with or down their concentration gradient is called passive diffusion. This allows the cell to use no energy to move the molecules. (Figure \(\PageIndex{4}\)). Some small molecules, like carbon dioxide, may cross the membrane bilayer directly by simple diffusion. However, charged molecules (including ions like Mg2+), as well as large molecules (think sugars), need the help of carriers or channels in the membrane. These structures ferry molecules across the membrane, a process known as facilitated diffusion (Figure \(\PageIndex{5}\)). Each molecule or group of similar molecules have their own specific carrier or channel protein to help it across the membrane and this allows for the cell to use no energy while still being selective about the molecules coming in and out. Osmosis as discussed before is usually a type of facilitated diffusion due to the use of a special water channel protein called an aquaporin.
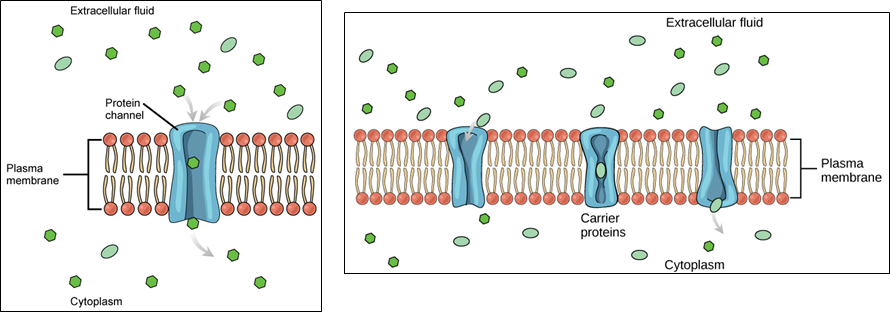
Active Transport
Another way to move molecules is via active transport. Active transport occurs when cells move molecules across the membrane against or "up" their concentration gradients (Figure \(\PageIndex{6}\)). A major difference between passive and active transport is that active transport requires adenosine triphosphate (ATP) or other forms of energy to move molecules “uphill.” Therefore, active transport structures are often called “pumps.”
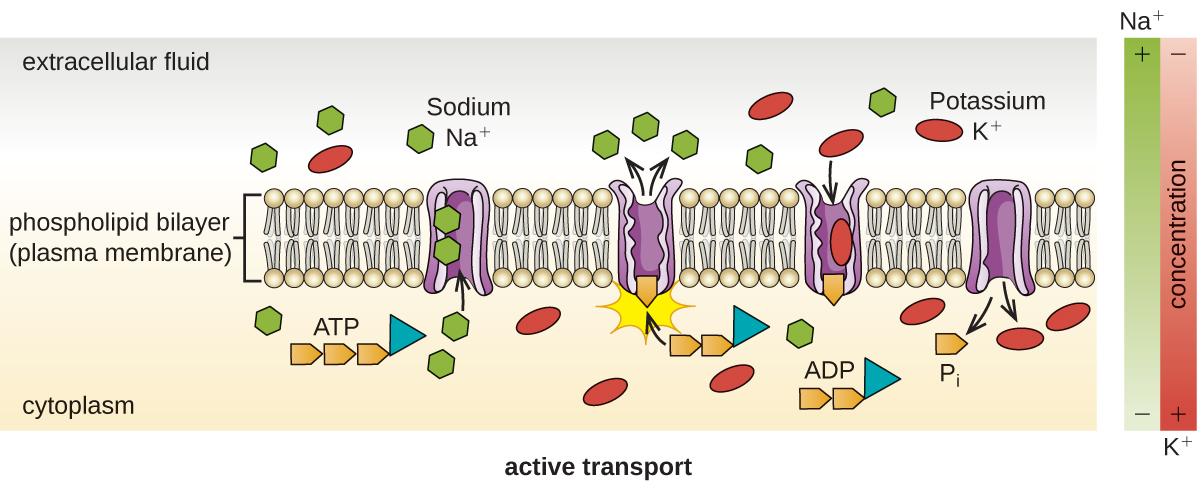
Group Translocation
Group translocation also transports substances into bacterial cells. In this case, as a molecule moves into a cell against its concentration gradient, it is chemically modified so that it does not require transport against an unfavorable concentration gradient. A common example of this is the bacterial phosphotransferase system, a series of carriers that phosphorylates (i.e., adds phosphate ions to) glucose or other sugars upon entry into cells. Since the phosphorylation of sugars is required during the early stages of sugar metabolism, the phosphotransferase system is considered to be an energy neutral system.
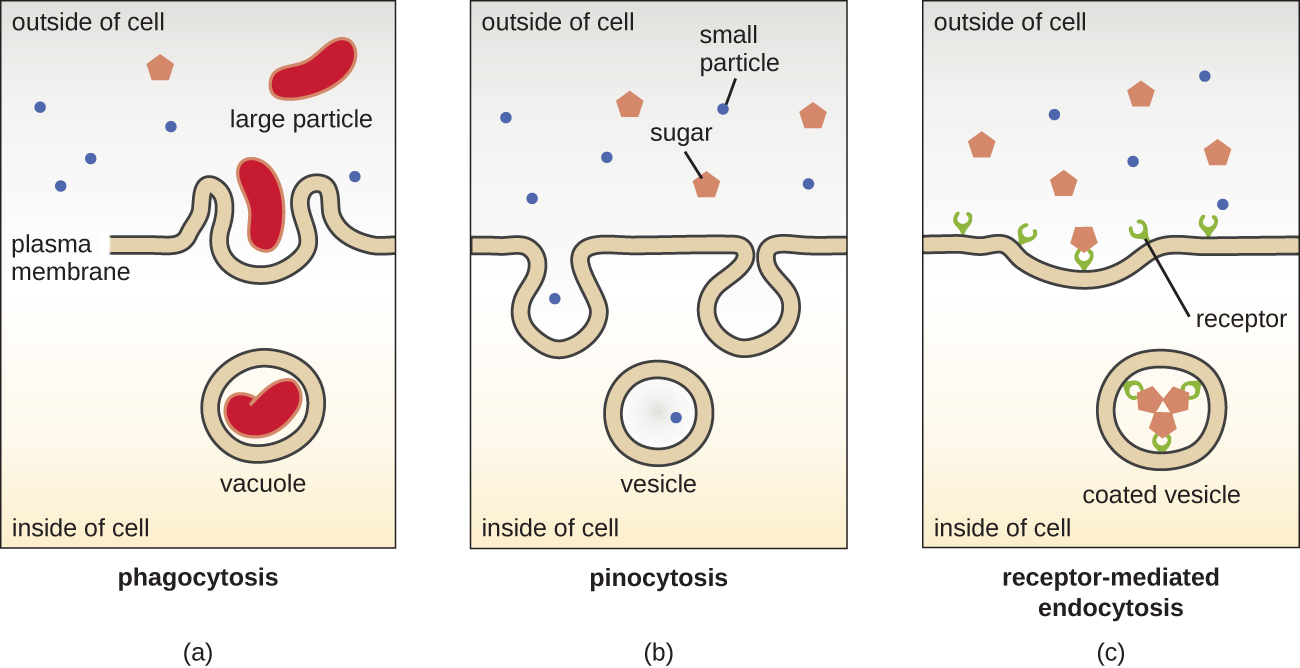
Eukaryotic Membrane Transport Mechanisms
The processes of simple diffusion, facilitated diffusion, and active transport are used in both eukaryotic and prokaryotic cells. However, eukaryotic cells also have the unique ability to perform various types of endocytosis, the uptake of matter through plasma membrane invagination and vacuole/vesicle formation (Figure \(\PageIndex{7}\)). A type of endocytosis involving the engulfment of large particles through membrane invagination is called phagocytosis, which means “cell eating.” In phagocytosis, particles (or other cells) are enclosed in a pocket within the membrane, which then pinches off from the membrane to form a vacuole that completely surrounds the particle. Another type of endocytosis is called pinocytosis, which means “cell drinking.” In pinocytosis, small, dissolved materials and liquids are taken into the cell through small vesicles. Saprophytic fungi, for example, obtain their nutrients from dead and decaying matter largely through pinocytosis.
Receptor-mediated endocytosis is a type of endocytosis that is initiated by specific molecules called ligands when they bind to cell surface receptors on the membrane. Receptor-mediated endocytosis is the mechanism that peptide and amine-derived hormones use to enter cells and is also used by various viruses and bacteria for entry into host cells.
The process by which secretory vesicles release their contents to the cell’s exterior is called exocytosis. Vesicles move toward the plasma membrane and then meld with the membrane, ejecting their contents out of the cell. Exocytosis is used by cells to remove waste products and may also be used to release chemical signals that can be taken up by other cells.
Key Concepts and Summary
- Halophiles require high salt concentration in the medium, whereas halotolerant organisms can grow and multiply in the presence of high salt but do not require it for growth.
- Halotolerant pathogens are an important source of foodborne illnesses because they contaminate foods preserved in salt.
- Barophiles require high atmospheric pressure.
- Some molecules can move across the bacterial membrane by simple diffusion. This is dependent on the concentration of the molecule inside the cell versus outside the cell.
- Water particularly is molecule that can be diffused across the membrane and its movement from out to in or in to out determines the osmotic pressure on the organism.
- Most large molecules must be actively transported through membrane structures using cellular energy.
- Photosynthetic bacteria depend on visible light for energy.
- Most bacteria, with few exceptions, require high moisture to grow.
Contributors and Attributions
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


