2.3: Carbon and Organic Molecules
- Page ID
- 75821
- Explain why carbon is important for life
- Identify common elements and structures found in organic molecules
- Explain the concept of isomerism
- Understand the synthesis of macromolecules
- Explain the importance and use of functional groups
- Explain dehydration (or condensation) and hydrolysis reactions
Cells are made of many complex molecules called macromolecules, such as proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules (any carbon-containing liquid, solid, or gas) that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
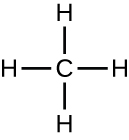
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula CH4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
Hydrocarbons
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH4) described above. We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which is released when these molecules are burned (oxidized). Methane, an excellent fuel, is the simplest hydrocarbon molecule, with a central carbon atom bonded to four different hydrogen atoms, as illustrated in Figure \(\PageIndex{1}\). The geometry of the methane molecule, where the atoms reside in three dimensions, is determined by the shape of its electron orbitals. The carbons and the four hydrogen atoms form a shape known as a tetrahedron, with four triangular faces; for this reason, methane is described as having tetrahedral geometry.
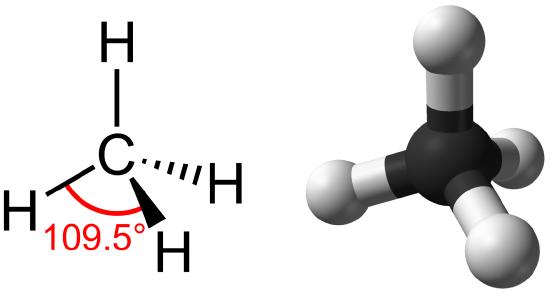
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, carbon rings, or combinations of both. Furthermore, individual carbon-to-carbon bonds may be single, double, or triple covalent bonds, and each type of bond affects the geometry of the molecule in a specific way. This three-dimensional shape or conformation of the large molecules of life (macromolecules) is critical to how they function.
Hydrocarbon Chains
Hydrocarbon chains are formed by successive bonds between carbon atoms and may be branched or unbranched. Furthermore, the overall geometry of the molecule is altered by the different geometries of single, double, and triple covalent bonds, illustrated in Figure \(\PageIndex{2}\). The hydrocarbons ethane, ethene, and ethyne serve as examples of how different carbon-to-carbon bonds affect the geometry of the molecule. The names of all three molecules start with the prefix “eth-,” which is the prefix for two carbon hydrocarbons. The suffixes “-ane,” “-ene,” and “-yne” refer to the presence of single, double, or triple carbon-carbon bonds, respectively. Thus, propane, propene, and propyne follow the same pattern with three carbon molecules, butane, butane, and butyne for four carbon molecules, and so on. Double and triple bonds change the geometry of the molecule: single bonds allow rotation along the axis of the bond, whereas double bonds lead to a planar configuration and triple bonds to a linear one. These geometries have a significant impact on the shape a particular molecule can assume.
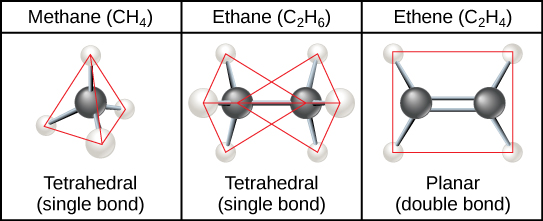
Hydrocarbon Rings
So far, the hydrocarbons we have discussed have been aliphatic hydrocarbons, which consist of linear chains of carbon atoms. Another type of hydrocarbon, aromatic hydrocarbons, consists of closed rings of carbon atoms. Ring structures are found in hydrocarbons, sometimes with the presence of double bonds, which can be seen by comparing the structure of cyclohexane to benzene in Figure \(\PageIndex{3}\). Examples of biological molecules that incorporate the benzene ring include some amino acids and cholesterol and its derivatives, including the hormones estrogen and testosterone. The benzene ring is also found in the herbicide 2,4-D. Benzene is a natural component of crude oil and has been classified as a carcinogen. Some hydrocarbons have both aliphatic and aromatic portions; beta-carotene is an example of such a hydrocarbon.
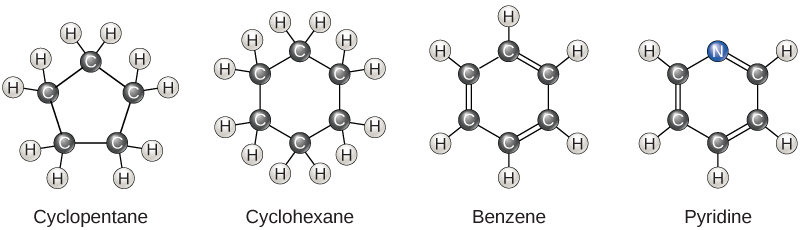
Biochemistry is the discipline that studies the chemistry of life, and its objective is to explain form and function based on chemical principles. Organic chemistry is the discipline devoted to the study of carbon-based chemistry, which is the foundation for the study of biomolecules and the discipline of biochemistry.
Other Elements in Living Cells
The most abundant element in cells is hydrogen (H), followed by carbon (C), oxygen (O), nitrogen (N), phosphorous (P), and sulfur (S). We call these elements macronutrients, and they account for about 99% of the dry weight of cells. Some elements, such as sodium (Na), potassium (K), magnesium (Mg), zinc (Zn), iron (Fe), calcium (Ca), molybdenum (Mo), copper (Cu), cobalt (Co), manganese (Mn), or vanadium (Va), are required by some cells in very small amounts and are called micronutrients or trace elements. All of these elements are essential to the function of many biochemical reactions, and, therefore, are essential to life.
The four most abundant elements in living matter (C, N, O, and H) have low atomic numbers and are thus light elements capable of forming strong bonds with other atoms to produce molecules (Figure \(\PageIndex{4}\)). Unlike carbon, nitrogen forms up to three bonds, oxygen forms up to two, and hydrogen forms one. When bonded together within molecules, oxygen, sulfur, and nitrogen often have one or more “lone pairs” of electrons that play important roles in determining many of the molecules’ physical and chemical properties. These traits in combination permit the formation of a vast number of diverse molecular species necessary to form the structures and enable the functions of living organisms.
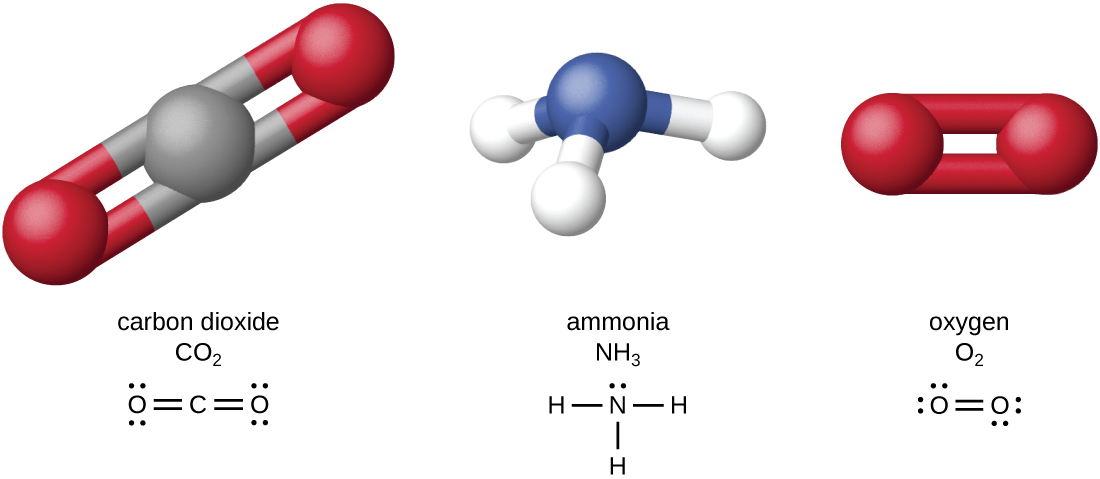
Living organisms contain inorganic compounds (mainly water and salts) and organic molecules. Organic molecules contain carbon; inorganic compounds do not. Carbon oxides and carbonates are exceptions; they contain carbon but are considered inorganic because they do not contain hydrogen. The atoms of an organic molecule are typically organized around chains of carbon atoms.
Inorganic compounds make up 1%–1.5% of a living cell’s mass. They are small, simple compounds that play important roles in the cell, although they do not form cell structures. Most of the carbon found in organic molecules originates from inorganic carbon sources such as carbon dioxide captured via carbon fixation by microorganisms.
- Describe the most abundant elements in nature.
- Describe the most abundant elements in nature.
- What are the differences between organic and inorganic molecules?
Organic Molecules
Organic molecules in organisms are generally larger and more complex than inorganic molecules. Their carbon skeletons are held together by covalent bonds. They form the cells of an organism and perform the chemical reactions that facilitate life. All of these molecules, called biomolecules because they are part of living matter, contain carbon, which is the building block of life. Carbon is a very unique element in that it has four valence electrons in its outer orbitals and can form four single covalent bonds with up to four other atoms at the same time. These atoms are usually oxygen, hydrogen, nitrogen, sulfur, phosphorous, and carbon itself; the simplest organic compound is methane, in which carbon binds only to hydrogen (Figure \(\PageIndex{5}\)).
As a result of carbon’s unique combination of size and bonding properties, carbon atoms can bind together in large numbers, thus producing a chain or carbon skeleton. The carbon skeleton of organic molecules can be straight, branched, or ring shaped (cyclic). Organic molecules are built on chains of carbon atoms of varying lengths; most are typically very long, which allows for a huge number and variety of compounds. No other element has the ability to form so many different molecules of so many different sizes and shapes.
Molecules with the same atomic makeup but different structural arrangement of atoms are called isomers. The concept of isomerism is very important in chemistry because the structure of a molecule is always directly related to its function. Slight changes in the structural arrangements of atoms in a molecule may lead to very different properties. Chemists represent molecules by their structural formula, which is a graphic representation of the molecular structure, showing how the atoms are arranged. Compounds that have identical molecular formulas but differ in the bonding sequence of the atoms are called structural isomers. The monosaccharides glucose, galactose, and fructose all have the same molecular formula, C6H12O6, but we can see from Figure \(\PageIndex{6}\) that the atoms are bonded together differently.
Isomers that differ in the spatial arrangements of atoms are called stereoisomers; one unique type is enantiomers. The properties of enantiomers were originally discovered by Louis Pasteur in 1848 while using a microscope to analyze crystallized fermentation products of wine. Enantiomers are molecules that have the characteristic of chirality, in which their structures are nonsuperimposable mirror images of each other. Chirality is an important characteristic in many biologically important molecules, as illustrated by the examples of structural differences in the enantiomeric forms of the monosaccharide glucose or the amino acid alanine (Figure \(\PageIndex{7}\)).
Many organisms are only able to use one enantiomeric form of certain types of molecules as nutrients and as building blocks to make structures within a cell. Some enantiomeric forms of amino acids have distinctly different tastes and smells when consumed as food. For example, L-aspartame, commonly called aspartame, tastes sweet, whereas D-aspartame is tasteless. Drug enantiomers can have very different pharmacologic affects. For example, the compound methorphan exists as two enantiomers, one of which acts as an antitussive (dextromethorphan, a cough suppressant), whereas the other acts as an analgesic (levomethorphan, a drug similar in effect to codeine).
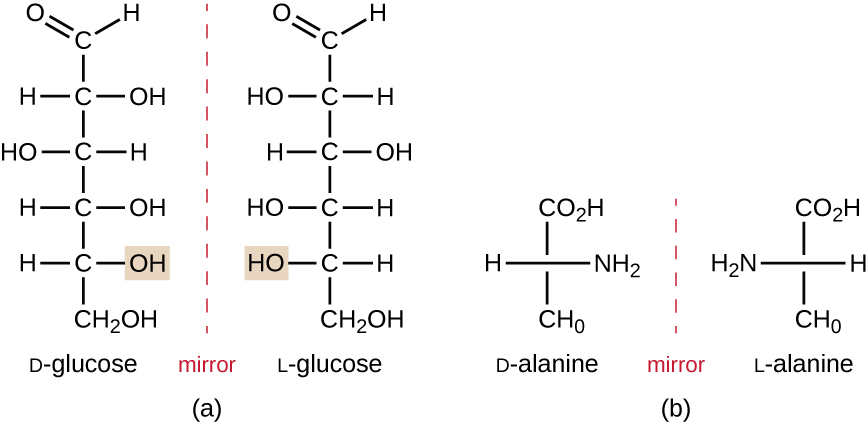
Enantiomers are also called optical isomers because they can rotate the plane of polarized light. Some of the crystals Pasteur observed from wine fermentation rotated light clockwise whereas others rotated the light counterclockwise. Today, we denote enantiomers that rotate polarized light clockwise (+) as d forms, and the mirror image of the same molecule that rotates polarized light counterclockwise (−) as the l form. The d and l labels are derived from the Latin words dexter (on the right) and laevus (on the left), respectively. These two different optical isomers often have very different biological properties and activities. Certain species of molds, yeast, and bacteria, such as Rhizopus, Yarrowia, and Lactobacillus spp., respectively, can only metabolize one type of optical isomer; the opposite isomer is not suitable as a source of nutrients. Another important reason to be aware of optical isomers is the therapeutic use of these types of chemicals for drug treatment, because some microorganisms can only be affected by one specific optical isomer.
- We say that life is carbon based. What makes carbon so suitable to be part of all the macromolecules of living organisms?
- Why is the arrangement of atoms so important?
Synthesis of Biological Molecules:
Biological macromolecules are large molecules, necessary for life, that are built from smaller organic molecules. There are four major classes of biological macromolecules (carbohydrates, lipids, proteins, and nucleic acids); each is an important cell component and performs a wide array of functions. Combined, these molecules make up the majority of a cell’s dry mass (recall that water makes up the majority of its complete mass). Biological macromolecules are organic, meaning they contain carbon. In addition, they may contain hydrogen, oxygen, nitrogen, and additional minor elements. They also share the use of functional groups and nearly identical building and deconstructing reactions.
Table \(\PageIndex{1}\): Functions of Macromolecules
| Macromolecule | Functions |
|---|---|
| Carbohydrates | Energy storage, receptors, food, structural role in plants, fungal cell walls, exoskeletons of insects |
| Lipids | Energy storage, membrane structure, insulation, hormones, pigments |
| Nucleic acids | Storage and transfer of genetic information |
| Proteins | Enzymes, structure, receptors, transport, structural role in the cytoskeleton of a cell and the extracellular matrix |
Biologically Significant Functional Groups
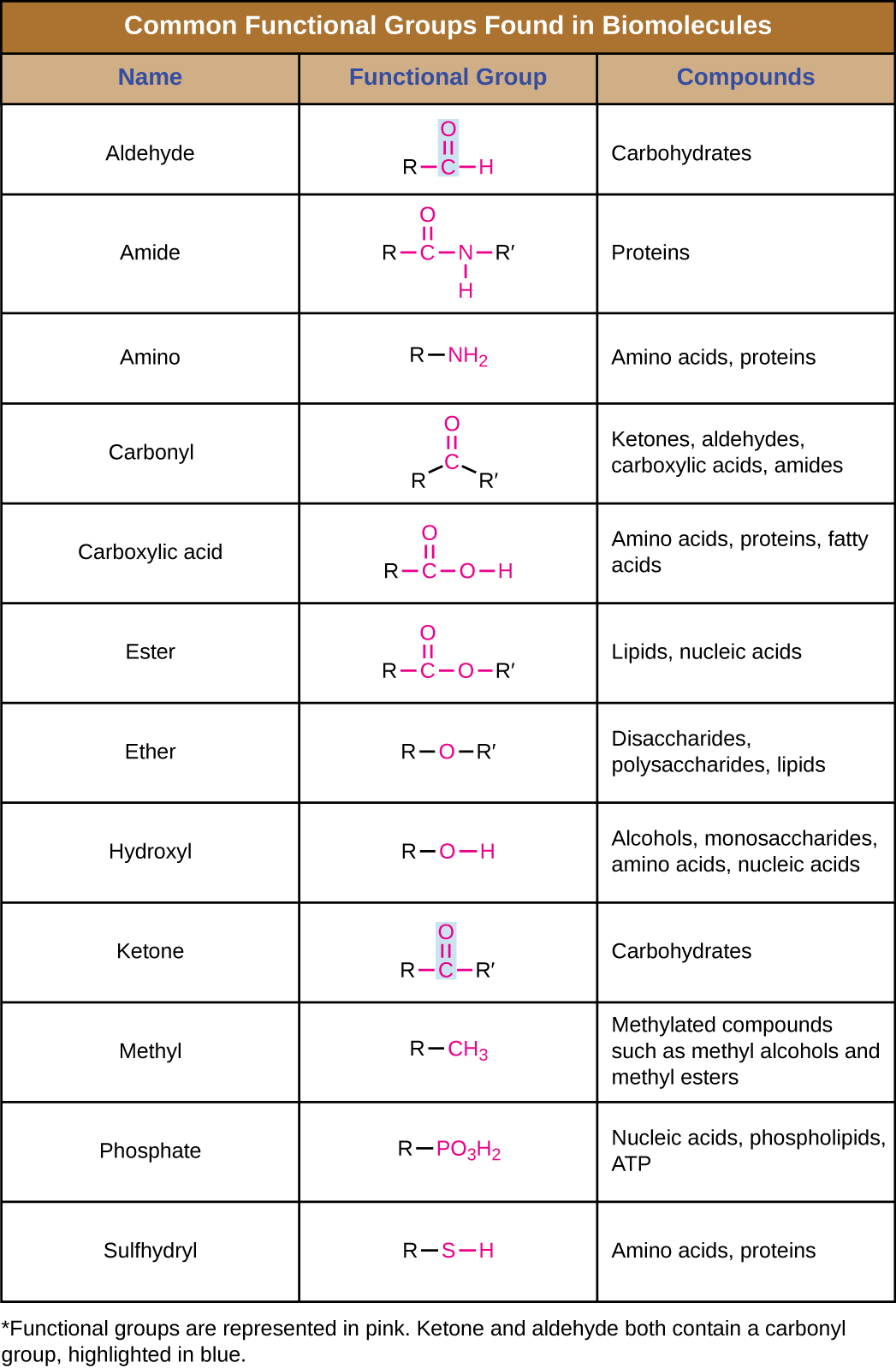
In addition to containing carbon atoms, biomolecules also contain functional groups—groups of atoms within molecules that are categorized by their specific chemical composition and the chemical reactions they perform, regardless of the molecule in which the group is found. Some of the most common functional groups are listed in Figure \(\PageIndex{8}\). In the formulas, the symbol R stands for “residue” and represents the remainder of the molecule. R might symbolize just a single hydrogen atom or it may represent a group of many atoms. Notice that some functional groups are relatively simple, consisting of just one or two atoms, while some comprise two of these simpler functional groups. For example, a carbonyl group is a functional group composed of a carbon atom double bonded to an oxygen atom: C=O. It is present in several classes of organic compounds as part of larger functional groups such as ketones, aldehydes, carboxylic acids, and amides. In ketones, the carbonyl is present as an internal group, whereas in aldehydes it is a terminal group.
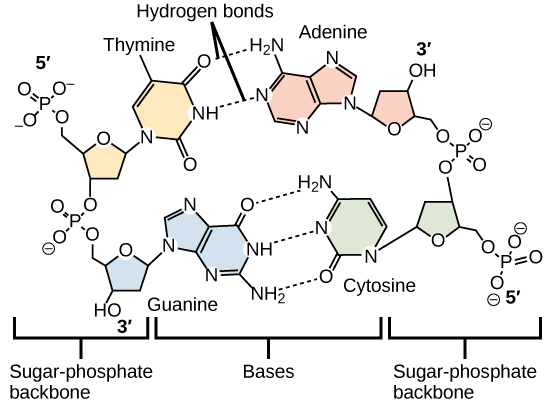
Hydrogen bonds between functional groups (within the same molecule or between different molecules) are important to the function of many macromolecules and help them to fold properly into and maintain the appropriate shape for functioning. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate, as illustrated in Figure \(\PageIndex{9}\).
Dehydration Synthesis
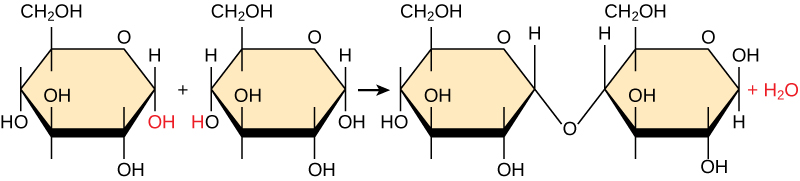
Most macromolecules are made from single subunits, or building blocks, called monomers. The monomers combine with each other using covalent bonds to form larger molecules known as polymers. In doing so, monomers release water molecules as byproducts. This type of reaction is known as dehydration synthesis, which means “to put together while losing water.”
In a dehydration synthesis reaction (Figure \(\PageIndex{10}\)), the hydrogen of one monomer combines with the hydroxyl group of another monomer, releasing a molecule of water. At the same time, the monomers share electrons and form covalent bonds. As additional monomers join, this chain of repeating monomers forms a polymer. Different types of monomers can combine in many configurations, giving rise to a diverse group of macromolecules. Even one kind of monomer can combine in a variety of ways to form several different polymers: for example, glucose monomers are the constituents of starch, glycogen, and cellulose.
Hydrolysis
Polymers are broken down into monomers in a process known as hydrolysis, which means “to split water,” a reaction in which a water molecule is used during the breakdown (Figure \(\PageIndex{11}\)). During these reactions, the polymer is broken into two components: one part gains a hydrogen atom (H+) and the other gains a hydroxyl molecule (OH–) from a split water molecule.

Dehydration and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes; dehydration reactions involve the formation of new bonds, requiring energy, while hydrolysis reactions break bonds and release energy. These reactions are similar for most macromolecules, but each monomer and polymer reaction is specific for its class. For example, in our bodies, food is hydrolyzed, or broken down, into smaller molecules by catalytic enzymes in the digestive system. This allows for easy absorption of nutrients by cells in the intestine. Each macromolecule is broken down by a specific enzyme. For instance, carbohydrates are broken down by amylase, sucrase, lactase, or maltase. Proteins are broken down by the enzymes pepsin and peptidase, and by hydrochloric acid. Lipids are broken down by lipases. Breakdown of these macromolecules provides energy for cellular activities.
 Visit this site to see visual representations of dehydration synthesis and hydrolysis.
Visit this site to see visual representations of dehydration synthesis and hydrolysis.
Key Concepts and Summary
- The unique properties of carbon make it a central part of biological molecules.Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function.
- Carbon has four electrons in its outermost shell and can form four bonds.
- Carbon and hydrogen can form hydrocarbon chains or rings. The shaping of these chains or rings define their overall chemical characteristics and function.
- The most abundant elements in cells are hydrogen, carbon, oxygen, nitrogen, phosphorus, and sulfur.
- Life is carbon based. Each carbon atom can bind to another one producing a carbon skeleton that can be straight, branched, or ring shaped.
- The same numbers and types of atoms may bond together in different ways to yield different molecules called isomers. Isomers may differ in the bonding sequence of their atoms (structural isomers) or in the spatial arrangement of atoms whose bonding sequences are the same (stereoisomers), and their physical and chemical properties may vary slightly or drastically.
- Proteins, carbohydrates, nucleic acids, and lipids are the four major classes of biological macromolecules—large molecules necessary for life that are built from smaller organic molecules.
- Functional groups confer specific chemical properties to molecules bearing them.
- Macromolecules are made up of single units known as monomers that are joined by covalent bonds to form larger polymers.
- A monomer joins with another monomer with the release of a water molecule, leading to the formation of a covalent bond. These types of reactions are known as dehydration or condensation reactions.
- When polymers are broken down into smaller units (monomers), a molecule of water is used for each bond broken by these reactions; such reactions are known as hydrolysis reactions.
- Dehydration and hydrolysis reactions are similar for all macromolecules, but each monomer and polymer reaction is specific to its class. Dehydration reactions typically require an investment of energy for new bond formation, while hydrolysis reactions typically release energy by breaking bonds.
Glossary
- aliphatic hydrocarbon
- hydrocarbon consisting of a linear chain of carbon atoms
- aromatic hydrocarbon
- hydrocarbon consisting of closed rings of carbon atoms
- biological macromolecule
- large molecule necessary for life that is built from smaller organic molecules
- dehydration synthesis
- (also, condensation) reaction that links monomer molecules together, releasing a molecule of water for each bond formed
- hydrocarbon
- molecule that consists only of carbon and hydrogen
- hydrolysis
- reaction causes breakdown of larger molecules into smaller molecules with the utilization of water
- monomer
- smallest unit of larger molecules called polymers
- organic molecule
- any molecule containing carbon (except carbon dioxide)
- polymer
- chain of monomer residues that is linked by covalent bonds; polymerization is the process of polymer formation from monomers by condensation
- substituted hydrocarbon
- hydrocarbon chain or ring containing an atom of another element in place of one of the backbone carbons
Contributors and Attributions
Connie Rye (East Mississippi Community College), Robert Wise (University of Wisconsin, Oshkosh), Vladimir Jurukovski (Suffolk County Community College), Jean DeSaix (University of North Carolina at Chapel Hill), Jung Choi (Georgia Institute of Technology), Yael Avissar (Rhode Island College) among other contributing authors. Original content by OpenStax (CC BY 4.0; Download for free at http://cnx.org/contents/185cbf87-c72...f21b5eabd@9.87).


