13.2: The Role of Microbiota in Human Physiology
- Page ID
- 51152
The cellular and genetic contribution to the human body it at least as large from its microbiota as from human cells. Our microbiota has been with humans since their evolutionary origins, and the interactions between the human body and its microbiota is finely tuned. It should not be surprising, therefore, that humans and their microbiota have developed mechanisms to understand and appropriately respond to each other’s chemical signals. The gut microbiota in particular is sometimes considered a “virtual organ” (https://doi.org/10.1038/sj.embor.7400731). The bacteria normally found in the gut produce many bioactive molecules that directly interact with endocrine, nervous, and immune systems, and the specific composition of the microbial community can have drastic effects on physiology. The human physiology reacts specifically to these chemical signals which can be indicative of nutritional state or potential infection. Conversely, adaptations in microbiota to the specialized environment of the human gut involved learning to use chemical cues from the body, such as the catecholamines, to modulate behavior according to the state of the host. Experiments in which the microbiota of lean and obese mice are swapped results in the swapping of phenotype (obese become lean and vice versa). Similar experiments have been done with calm and anxious mice.
Short Chain Fatty Acids (SCFAs)
Possibly one of the most physiologically active groups of “communication” molecules produced by the gut microbiota are the short chain fatty acids (SCFAs) which are waste products of certain bacterial fermentations. Gut bacteria in the Bacteroidetes and Firmicutes phyla are the main producers of the SCFAs acetate, butyrate, and propionate through fermentation of non-digestible carbohydrates. The presence of SCFAs therefore indicates the metabolic activity and taxonomic composition of the gut microbiota and indirectly the nutritive value of the food which has been consumed. This may partially explain the link between increased dietary fiber and improved health outcomes considering the demonstrated effects of SCFAs on both glucose and lipid metabolism.
Bacterially-produced SCFAs in the colon positively impact glucose handling in the body through multiple routes (Fig. 9). In one mechanism, SCFAs activate specific receptors on the surface of endocrine cells within the intestinal epithelium. In response, these endocrine cells produce two hormones (peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) (Figure \(\PageIndex{1}\)). These hormones stimulate glucose uptake by adipose and muscle cells, increase insulin production, and decreases glucagon production by the pancreas. SCFAs also enter the bloodstream directly decrease gluconeogenesis in the liver. The combined effect of SCFAs is therefore to decrease plasma glucose levels and subsequently decreased risk of diabetes (Figure \(\PageIndex{1}\)). Both PYY and GLP-1 have also been shown to promote satiety.
In addition to reducing plasma glucose levels, SCFAs also regulate lipid metabolism through the interaction with the same endocrine receptors in other parts of the body. Specifically, SCFAs reduce free fatty acids in blood plasma, increase fatty acid oxidation in liver and muscle tissue, and reduce plasma cholesterol levels (Figure \(\PageIndex{1}\)). Because of these beneficial effects on glucose, fatty acid, and cholesterol levels, altering the gut microbiota or diet to increase the production of SCFAs has been suggested as a way to decrease the risk of type II diabetes and cardiovascular disease.
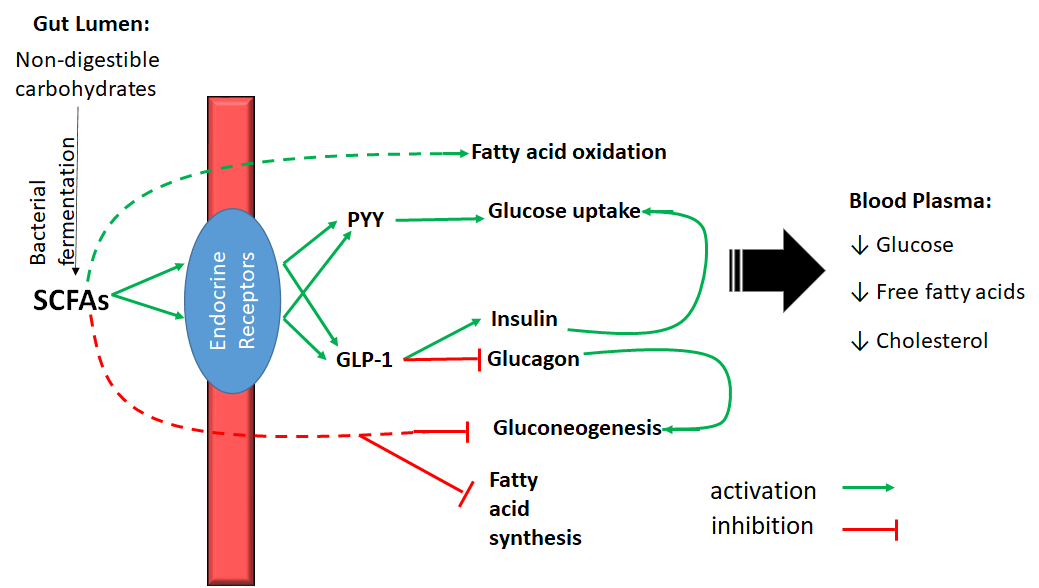
The role of SCFAs in metabolic physiology might be expected due to their ability to be utilized as carbon and energy sources and their chemical relationship to intermediates of lipid metabolism. The influence of SCFAs on the production of the neurotransmitter serotonin is more surprising. Serotonin can affect a range of behaviors and physiological functions including mood and social behavior, appetite and digestion, sleep, memory, and sexual desire and function. Although serotonin is typically associated with actions of the central nervous system (CNS), the vast majority of serotonin (80-90%) is produced in the intestines. SCFAs increase transcription of the gene for the critical enzyme in serotonin synthesis. Microbially-produced SCFAs, therefore, may play a critical role in production and homeostasis of intestinal serotonin. In addition, many bacteria in the gut can also synthesize serotonin themselves. The impact of gut microbiota on serotonin production may be part of their communication with the CNS, sometimes referred to as the brain-gut-microbiome axis.
Microbiota and Behavior
Among an increasing number of studies examining the brain-gut-microbiome axis, communication between the gut microbiota and the CNS and its effect on behavior was effectively demonstrated in a study by Bravo et al. in 2011. In this study, mice fed the probiotic bacterium Lactobacillus rhamnosus JB-1 for 28 days displayed a significant decrease in depression- and anxiety-like behavior compared to controls, but these effects were eliminated upon severing the vagus nerve, which connects the digestive tract to the CNS. In addition, mice had gene express in their brains consistent with the mood modifying effects of JB-1. Although the specific chemical signaling pathway between JB-1 and the CNS was not determined in this study, communication is clearly occurring through the vagus nerve, although modified levels of serotonin may play a role.
Communication between the gut microbiota and the CNS not only affect its functioning, but its development as well. A comparison between the gene expression in the developing brains of germ-free and conventional mice showed different expression in almost 200 genes. These gene expression analyses in combination with behavioral assessments indicate a substantial link between the presence of normal gut microbiota in an infant and development of motor control and anxiety-related brain systems. As with metabolic disorders, altering the gut microbiota has been suggested as a potential treatment for several mental health conditions from anxiety and depression to autism.
Protection against Infection
A well-established normal flora protects us from infection (Figure \(\PageIndex{2}\)), particularly (but not exclusively) from pathogens which infect the GI tract. Normal flora are critical for the proper development and functioning of the immune system. By contributing to a robust immune system the normal flora help protect us from all varieties of pathogens. The normal flora can protect us more directly as well.
First, the normal flora physically and nutritionally don’t leave room for pathogens to establish. This is similar to the way that weeds can’t grow in a well-established lawn. There simply isn’t room. If the lawn is damaged, however, weeds will establish quickly and vigorously. One good example of this is Clostridium botulinum. Children under the age of about 1 year do not yet have a robust enough normal flora and can be infected with C. botulinum leading to infant botulism. One common source of these infections is honey, but C. botulinum endospores could potentially be found anywhere. Older children and adults with established flora cannot get an intestinal infection with C. botulinum. Another Clostridium species, C. difficile can cause serious infection in anyone whose normal flora has been disrupted. This is normally a nosocomial infection associated with long term use of broad-spectrum antibiotics. C. difficile is more antibiotic-resistant than most gut bacteria, and with the other bacteria eliminated it can flourish. This situation, where an infection occurs in addition to an already-existing infection (often as a result of the treatment of the initial infection), is sometimes referred to as a superinfection. C. difficile produces exotoxins which kills the lining of the intestines which then sloughs off in the feces in sheets. This sometimes fatal infection can cause severe diarrhea, dehydration, and eventually perforation of the intestines. Because the infection was initiated by a lack of normal flora, over the past decade or so fecal transplants have become a relatively common treatment for the most severe C. difficile infections.
Second, bacteria of the normal flora can produce specific compounds which target potential pathogens. These compounds might be antibiotics, but other compounds can also have an inhibitory effect on potential pathogens. For instance, Streptococcus mutans and the fungus Candida both normally inhabit the mouth. In its virulent form, Candida cause overgrow in the mouth causing thrush. The S. mutans autoinducer CSP (competence stimulating factor) prevents Candida from transitioning to its virulent form and holds this potential pathogen in check. A similar interaction between Streptococci and Candida occurs in the vagina. When antibiotic treatment decreases the population of Streptococcus, the Candida can overgrow and cause a yeast infection.
Finally, SCFAs have been shown to prevent to growth of enteric pathogens such as E. coli O157:H7.
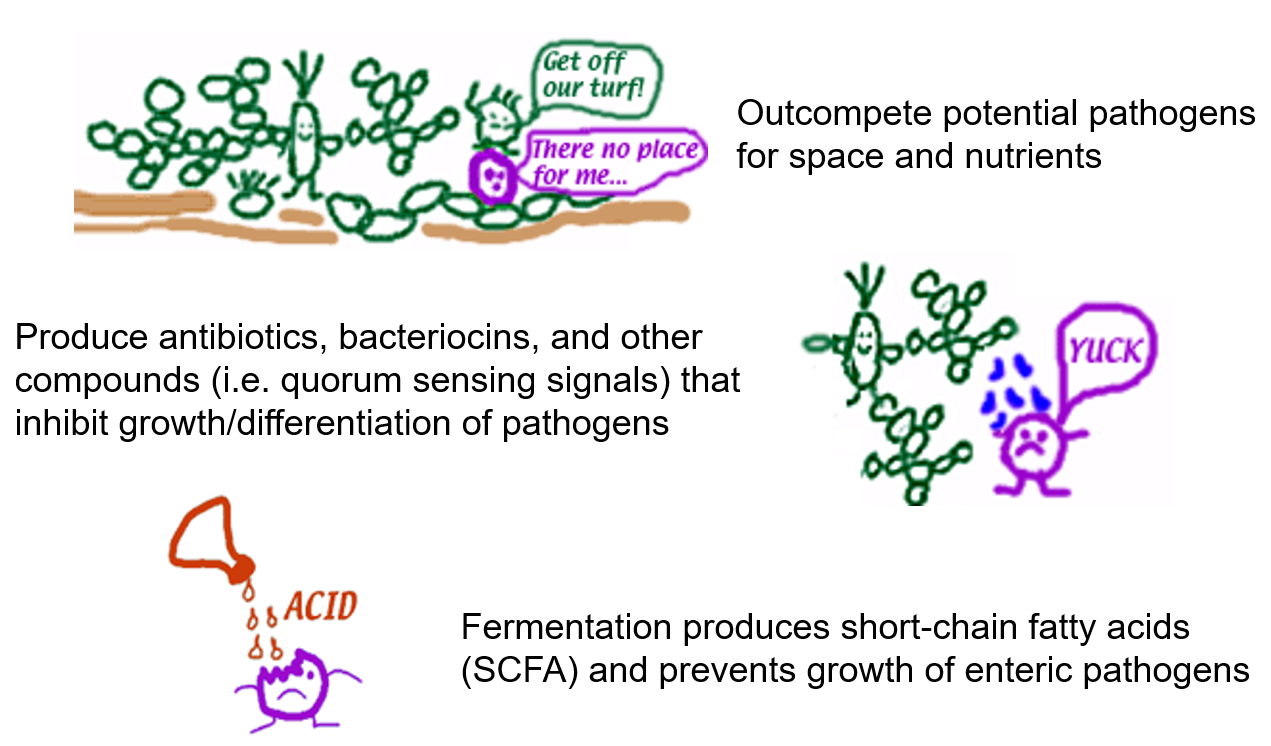
Figure \(\PageIndex{2}\): Role of normal flora in fighting infection. (adapted from resources found at outreach.colorado.edu)

