7.2.1: Controlling Microbial Growth
- Page ID
- 42502
Learning Objectives
- Compare disinfectants, antiseptics, and sterilants
- Describe the principles of controlling the presence of microorganisms through sterilization and disinfection
- Differentiate between microorganisms of various biological safety levels and explain methods used for handling microbes at each level
Clinical Focus: Part 1
Roberta is a 46-year-old real estate agent who recently underwent a cholecystectomy (surgery to remove painful gallstones). The surgery was performed laparoscopically with the aid of a duodenoscope, a specialized endoscope that allows surgeons to see inside the body with the aid of a tiny camera. On returning home from the hospital, Roberta developed abdominal pain and a high fever. She also experienced a burning sensation during urination and noticed blood in her urine. She notified her surgeon of these symptoms, per her postoperative instructions.
Exercise \(\PageIndex{1}\)
What are some possible causes of Roberta’s symptoms?
To prevent the spread of human disease, it is necessary to control the growth and abundance of microbes in or on various items frequently used by humans. Inanimate items, such as doorknobs, toys, or towels, which may harbor microbes and aid in disease transmission, are called fomites. Two factors heavily influence the level of cleanliness required for a particular fomite and, hence, the protocol chosen to achieve this level. The first factor is the application for which the item will be used. For example, invasive applications that require insertion into the human body require a much higher level of cleanliness than applications that do not. The second factor is the level of resistance to antimicrobial treatment by potential pathogens. For example, foods preserved by canning often become contaminated with the bacterium Clostridium botulinum, which produces the neurotoxin that causes botulism. Because C. botulinum can produce endospores that can survive harsh conditions, extreme temperatures and pressures must be used to eliminate the endospores. Other organisms may not require such extreme measures and can be controlled by a procedure such as washing clothes in a laundry machine.
Sterilization
The most extreme protocols for microbial control aim to achieve sterilization: the complete removal or killing of all vegetative cells, endospores, and viruses from the targeted item or environment. Sterilization protocols are generally reserved for laboratory, medical, manufacturing, and food industry settings, where it may be imperative for certain items to be completely free of potentially infectious agents. Sterilization can be accomplished through either physical means, such as exposure to high heat, pressure, or filtration through an appropriate filter, or by chemical means. Chemicals that can be used to achieve sterilization are called sterilants. Sterilants effectively kill all microbes and viruses, and, with appropriate exposure time, can also kill endospores.
For many clinical purposes, aseptic technique is necessary to prevent contamination of sterile surfaces. Aseptic technique involves a combination of protocols that collectively maintain sterility, or asepsis, thus preventing contamination of the patient with microbes and infectious agents. Failure to practice aseptic technique during many types of clinical procedures may introduce microbes to the patient’s body and put the patient at risk for sepsis, a systemic inflammatory response to an infection that results in high fever, increased heart and respiratory rates, shock, and, possibly, death. Medical procedures that carry risk of contamination must be performed in a sterile field, a designated area that is kept free of all vegetative microbes, endospores, and viruses. Sterile fields are created according to protocols requiring the use of sterilized materials, such as packaging and drapings, and strict procedures for washing and application of sterilants. Other protocols are followed to maintain the sterile field while the medical procedure is being performed.
One food sterilization protocol, commercial sterilization, uses heat at a temperature low enough to preserve food quality but high enough to destroy common pathogens responsible for food poisoning, such as C. botulinum. Because C. botulinum and its endospores are commonly found in soil, they may easily contaminate crops during harvesting, and these endospores can later germinate within the anaerobic environment once foods are canned. Metal cans of food contaminated with C. botulinum will bulge due to the microbe’s production of gases; contaminated jars of food typically bulge at the metal lid. To eliminate the risk for C. botulinum contamination, commercial food-canning protocols are designed with a large margin of error. They assume an impossibly large population of endospores (1012 per can) and aim to reduce this population to 1 endospore per can to ensure the safety of canned foods. For example, low- and medium-acid foods are heated to 121 °C for a minimum of 2.52 minutes, which is the time it would take to reduce a population of 1012 endospores per can down to 1 endospore at this temperature. Even so, commercial sterilization does not eliminate the presence of all microbes; rather, it targets those pathogens that cause spoilage and foodborne diseases, while allowing many nonpathogenic organisms to survive. Therefore, “sterilization” is somewhat of a misnomer in this context, and commercial sterilization may be more accurately described as “quasi-sterilization.”
Exercise \(\PageIndex{3}\)
What is the difference between sterilization and aseptic technique?
The Association of Surgical Technologists publishes standards for aseptic technique, including creating and maintaining a sterile field.
Other Methods of Control
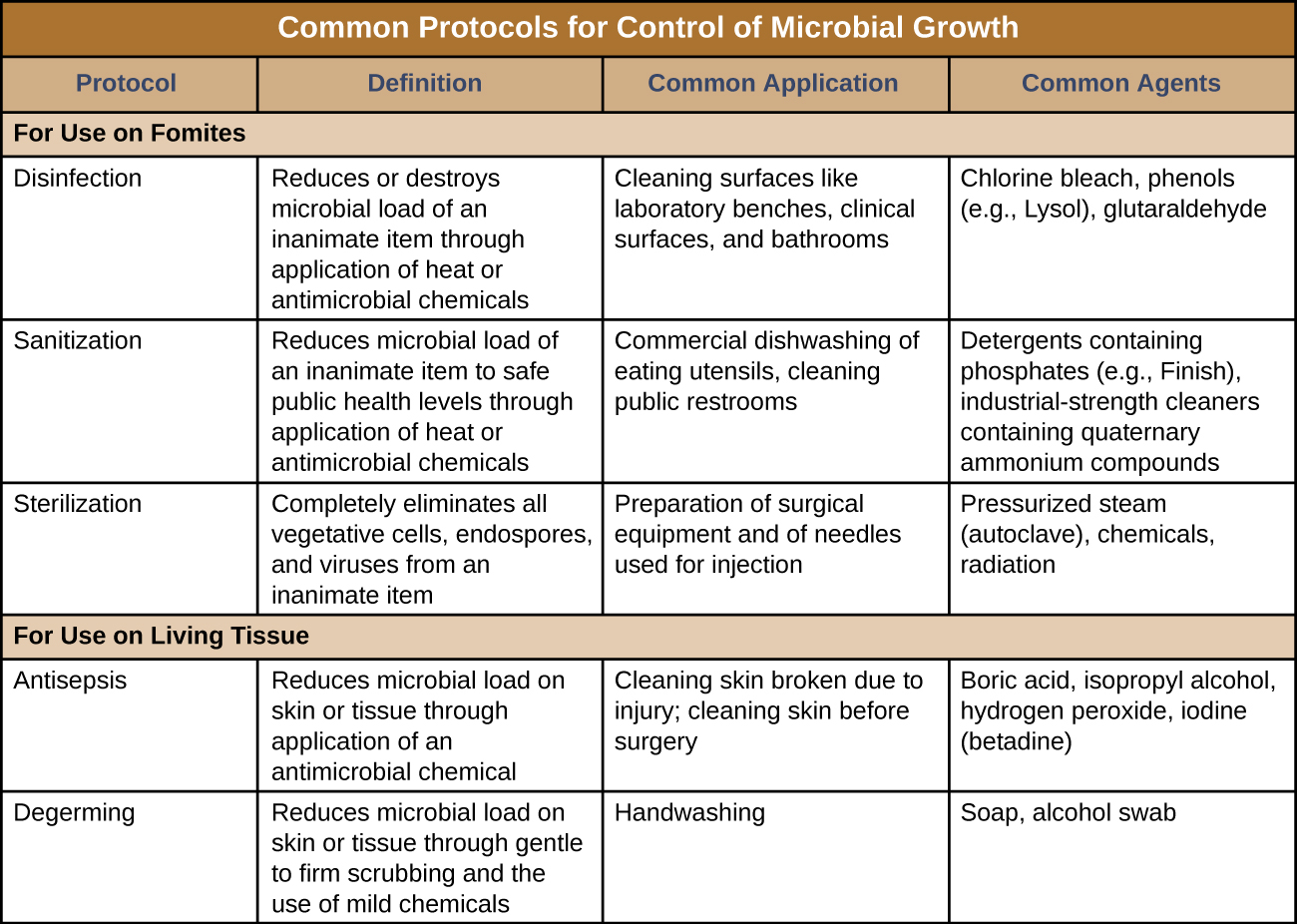
Sterilization protocols require procedures that are not practical, or necessary, in many settings. Various other methods are used in clinical and nonclinical settings to reduce the microbial load on items. Although the terms for these methods are often used interchangeably, there are important distinctions (Figure \(\PageIndex{3}\)).
The process of disinfection inactivates most microbes on the surface of a fomite by using antimicrobial chemicals or heat. Because some microbes remain, the disinfected item is not considered sterile. Ideally, disinfectants should be fast acting, stable, easy to prepare, inexpensive, and easy to use. An example of a natural disinfectant is vinegar; its acidity kills most microbes. Chemical disinfectants, such as chlorine bleach or products containing chlorine, are used to clean nonliving surfaces such as laboratory benches, clinical surfaces, and bathroom sinks. Typical disinfection does not lead to sterilization because endospores tend to survive even when all vegetative cells have been killed.
Unlike disinfectants, antiseptics are antimicrobial chemicals safe for use on living skin or tissues. Examples of antiseptics include hydrogen peroxide and isopropyl alcohol. The process of applying an antiseptic is called antisepsis. In addition to the characteristics of a good disinfectant, antiseptics must also be selectively effective against microorganisms and able to penetrate tissue deeply without causing tissue damage.
The type of protocol required to achieve the desired level of cleanliness depends on the particular item to be cleaned. For example, those used clinically are categorized as critical, semicritical, and noncritical. Critical items must be sterile because they will be used inside the body, often penetrating sterile tissues or the bloodstream; examples of critical items include surgical instruments, catheters, and intravenous fluids. Gastrointestinal endoscopes and various types of equipment for respiratory therapies are examples of semicritical items; they may contact mucous membranes or nonintact skin but do not penetrate tissues. Semicritical items do not typically need to be sterilized but do require a high level of disinfection. Items that may contact but not penetrate intact skin are noncritical items; examples are bed linens, furniture, crutches, stethoscopes, and blood pressure cuffs. These articles need to be clean but not highly disinfected.
The act of handwashing is an example of degerming, in which microbial numbers are significantly reduced by gently scrubbing living tissue, most commonly skin, with a mild chemical (e.g., soap) to avoid the transmission of pathogenic microbes. Wiping the skin with an alcohol swab at an injection site is another example of degerming. These degerming methods remove most (but not all) microbes from the skin’s surface.
The term sanitization refers to the cleansing of fomites to remove enough microbes to achieve levels deemed safe for public health. For example, commercial dishwashers used in the food service industry typically use very hot water and air for washing and drying; the high temperatures kill most microbes, sanitizing the dishes. Surfaces in hospital rooms are commonly sanitized using a chemical disinfectant to prevent disease transmission between patients. Figure \(\PageIndex{3}\) summarizes common protocols, definitions, applications, and agents used to control microbial growth.
Exercise \(\PageIndex{4}\)
- What is the difference between a disinfectant and an antiseptic?
- Which is most effective at removing microbes from a product: sanitization, degerming, or sterilization? Explain.
Clinical Focus: Part 2
Roberta’s physician suspected that a bacterial infection was responsible for her sudden-onset high fever, abdominal pain, and bloody urine. Based on these symptoms, the physician diagnosed a urinary tract infection (UTI). A wide variety of bacteria may cause UTIs, which typically occur when bacteria from the lower gastrointestinal tract are introduced to the urinary tract. However, Roberta’s recent gallstone surgery caused the physician to suspect that she had contracted a nosocomial (hospital-acquired) infection during her surgery. The physician took a urine sample and ordered a urine culture to check for the presence of white blood cells, red blood cells, and bacteria. The results of this test would help determine the cause of the infection. The physician also prescribed a course of the antibiotic ciprofloxacin, confident that it would clear Roberta’s infection.
Exercise \(\PageIndex{5}\)
What are some possible ways that bacteria could have been introduced to Roberta’s urinary tract during her surgery?
Measuring Microbial Control
Physical and chemical methods of microbial control that kill the targeted microorganism are identified by the suffix -cide (or -cidal). The prefix indicates the type of microbe or infectious agent killed by the treatment method: bactericides kill bacteria, viricides kill or inactivate viruses, and fungicides kill fungi. Other methods do not kill organisms but, instead, stop their growth, making their population static; such methods are identified by the suffix -stat (or -static). For example, bacteriostatic treatments inhibit the growth of bacteria, whereas fungistatic treatments inhibit the growth of fungi. Factors that determine whether a particular treatment is -cidal or -static include the types of microorganisms targeted, the concentration of the chemical used, and the nature of the treatment applied.
Although -static treatments do not actually kill infectious agents, they are often less toxic to humans and other animals, and may also better preserve the integrity of the item treated. Such treatments are typically sufficient to keep the microbial population of an item in check. The reduced toxicity of some of these -static chemicals also allows them to be impregnated safely into plastics to prevent the growth of microbes on these surfaces. Such plastics are used in products such as toys for children and cutting boards for food preparation. When used to treat an infection, -static treatments are typically sufficient in an otherwise healthy individual, preventing the pathogen from multiplying, thus allowing the individual’s immune system to clear the infection.
The degree of microbial control can be evaluated using a microbial death curve to describe the progress and effectiveness of a particular protocol. When exposed to a particular microbial control protocol, a fixed percentage of the microbes within the population will die. Because the rate of killing remains constant even when the population size varies, the percentage killed is more useful information than the absolute number of microbes killed. Death curves are often plotted as semilog plots just like microbial growth curves because the reduction in microorganisms is typically logarithmic (Figure \(\PageIndex{4}\)). The amount of time it takes for a specific protocol to produce a one order-of-magnitude decrease in the number of organisms, or the death of 90% of the population, is called the decimal reduction time (DRT) or D-value.
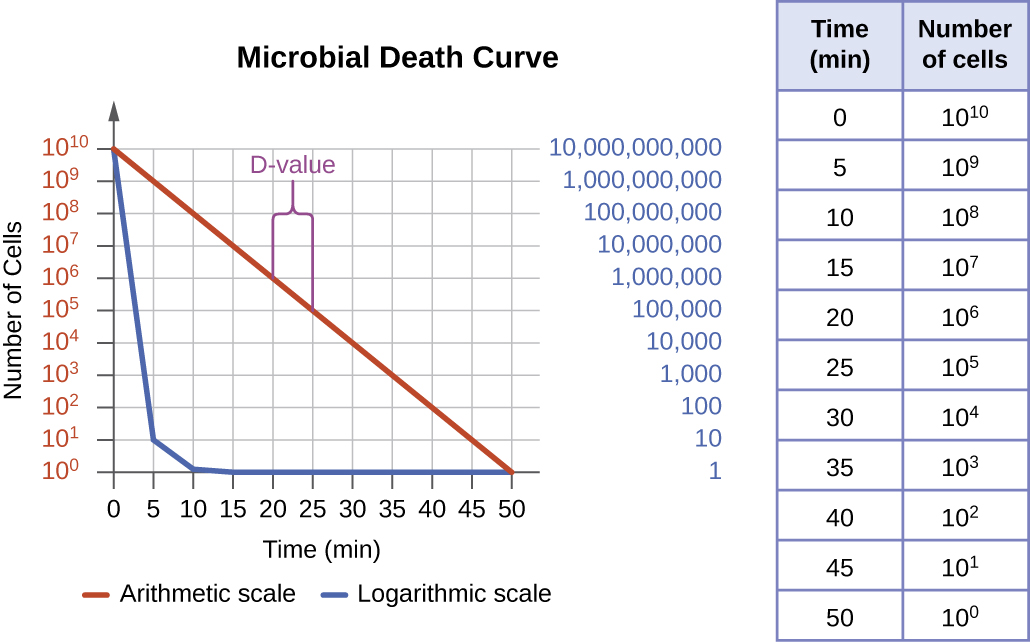
Several factors contribute to the effectiveness of a disinfecting agent or microbial control protocol. First, as demonstrated in Figure \(\PageIndex{4}\), the length of time of exposure is important. Longer exposure times kill more microbes. Because microbial death of a population exposed to a specific protocol is logarithmic, it takes longer to kill a high-population load than a low-population load exposed to the same protocol. A shorter treatment time (measured in multiples of the D-value) is needed when starting with a smaller number of organisms. Effectiveness also depends on the susceptibility of the agent to that disinfecting agent or protocol. The concentration of disinfecting agent or intensity of exposure is also important. For example, higher temperatures and higher concentrations of disinfectants kill microbes more quickly and effectively. Conditions that limit contact between the agent and the targeted cells cells—for example, the presence of bodily fluids, tissue, organic debris (e.g., mud or feces), or biofilms on surfaces—increase the cleaning time or intensity of the microbial control protocol required to reach the desired level of cleanliness. All these factors must be considered when choosing the appropriate protocol to control microbial growth in a given situation.
Exercise \(\PageIndex{6}\)
- What are two possible reasons for choosing a bacteriostatic treatment over a bactericidal one?
- Name at least two factors that can compromise the effectiveness of a disinfecting agent.
Key Concepts and Summary
- Inanimate items that may harbor microbes and aid in their transmission are called fomites. The level of cleanliness required for a fomite depends both on the item’s use and the infectious agent with which the item may be contaminated.
- Disinfection removes potential pathogens from a fomite, whereas antisepsis uses antimicrobial chemicals safe enough for tissues; in both cases, microbial load is reduced, but microbes may remain unless the chemical used is strong enough to be a sterilant.
- The amount of cleanliness (sterilization versus high-level disinfection versus general cleanliness) required for items used clinically depends on whether the item will come into contact with sterile tissues (critical item), mucous membranes (semicritical item), or intact skin (noncritical item).
- Medical procedures with a risk for contamination should be carried out in a sterile field maintained by proper aseptic technique to prevent sepsis.
- Sterilization is necessary for some medical applications as well as in the food industry, where endospores of Clostridium botulinum are killed through commercial sterilization protocols.
- Physical or chemical methods to control microbial growth that result in death of the microbe are indicated by the suffixes -cide or -cidal (e.g., as with bactericides, viricides, and fungicides), whereas those that inhibit microbial growth are indicated by the suffixes -stat or-static (e.g., bacteriostatic, fungistatic).
- Microbial death curves display the logarithmic decline of living microbes exposed to a method of microbial control. The time it takes for a protocol to yield a 1-log (90%) reduction in the microbial population is the decimal reduction time, or D-value.
- When choosing a microbial control protocol, factors to consider include the length of exposure time, the type of microbe targeted, its susceptibility to the protocol, the intensity of the treatment, the presence of organics that may interfere with the protocol, and the environmental conditions that may alter the effectiveness of the protocol.
Footnotes
- 1 US Centers for Disease Control and Prevention. “Recognizing the Biosafety Levels.” http://www.cdc.gov/training/quicklearns/biosafety/. Accessed June 7, 2016.
Contributors and Attributions
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


