6.2: Fermentation
- Page ID
- 42515
Learning Objectives
- Define fermentation and explain why it does not require oxygen
- Describe the fermentation pathways and their end products and give examples of microorganisms that use these pathways
- Compare and contrast fermentation and respiration
There are two mechanisms by which chemoheterotrophs can generate ATP: respiration and fermentation. Although respiration relies on the generation of a proton gradient and ATP synthesis by oxidative phosphorylation, ATP synthesis in fermentation is entirely through substrate-level phosphorylation in metabolic pathways. In general, the amount of ATP produced through fermentation is less than respiration, but there are situations where fermentation is necessary or preferable. Many prokaryotes, such as E. coli, are facultative, meaning that should the environmental conditions change to provide an appropriate inorganic final electron acceptor for respiration, organisms containing all the genes required to do so will switch to respiration because respiration allows for much greater ATP production. Whereas lack of an appropriate inorganic final electron acceptor is environmentally dependent, some organisms lack the ability to respire altogether. Many prokaryotes, including members of the clinically important genera Streptococcus and Clostridium, rely entirely on fermentation for ATP generation.
If respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for glycolysis and other catabolic pathways to continue. Some living systems use a metabolite produced through the cell's metabolite (such as pyruvate) as a final electron acceptor through a process called fermentation. Because all the NADH produced must be reoxidized to NAD+, the net NADH of any fermentation pathway must be zero (0). Considering that the purpose of fermentation is to generate ATP, there must also be a net gain of ATP in these metabolic pathways.
Fermentation does not involve an electron transport chain and does not directly produce any additional ATP beyond that produced during glycolysis by substrate-level phosphorylation. Organisms carrying out fermentation typically produce a maximum of two ATP molecules per glucose during glycolysis. Table \(\PageIndex{1}\) compares the final electron acceptors and methods of ATP synthesis in aerobic respiration, anaerobic respiration, and fermentation. Note that the number of ATP molecules shown for glycolysis assumes the Embden-Meyerhof-Parnas pathway. The number of ATP molecules made by substrate-level phosphorylation (SLP) versus oxidative phosphorylation (OP) are indicated.
| Type of Metabolism | Example | Final Electron Acceptor | Pathways Involved in ATP Synthesis (Type of Phosphorylation) | Maximum Yield of ATP Molecules |
|---|---|---|---|---|
| Aerobic respiration | Pseudomonas aeruginosa | O2 |
EMP glycolysis (SLP) Krebs cycle (SLP) Electron transport and chemiosmosis (OP): |
2 2 34 |
| Total | 38 | |||
| Anaerobic respiration | Paracoccus denitrificans |
NO3−,SO−24,Fe+3 other inorganics |
EMP glycolysis (SLP) Krebs cycle (SLP) Electron transport and chemiosmosis (OP): |
2 2 1–32 |
| Total | 5–36 | |||
| Fermentation | Candida albicans |
Organics (usually pyruvate) |
EMP glycolysis (SLP) Fermentation |
2 0 |
| Total | 2 |
In all bacterial fermentations, at least one of the waste products produced is an organic acid. This feature of bacterial fermentations is frequently exploited in metabolic tests used to identify bacteria. For example, E. coli can ferment lactose, forming gas, whereas some of its close Gram-negative relatives cannot. The ability to ferment the sugar alcohol sorbitol is used to identify the pathogenic enterohemorrhagic O157:H7 strain of E. coli because, unlike other E. coli strains, it is unable to ferment sorbitol. Last, mannitol fermentation differentiates the mannitol-fermenting Staphylococcus aureus from other non–mannitol-fermenting staphylococci.
The simplest fermentation, which is used by some bacteria, like those in yogurt and other soured food products, and by animals in muscles during oxygen depletion, is homolactic or lactic acid fermentation (Figure \(\PageIndex{1}\). In homolactic fermentation the electrons on NADH produced during glycolysis are reoxidized to NAD+ by donating their electrons to the end product of glycolysis, pyruvate. The resulting waste product is lactate (lactic acid).
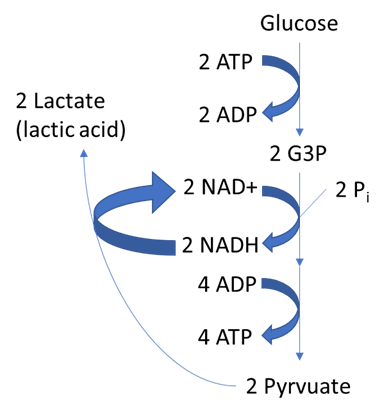
Bacteria of several Gram-positive genera, including Lactobacillus, Leuconostoc, and Streptococcus, are collectively known as the lactic acid bacteria (LAB), and various strains are important in food production. During yogurt and cheese production, the highly acidic environment generated by lactic acid fermentation denatures proteins contained in milk, causing it to solidify. When lactic acid is the only fermentation product, the process is said to be homolactic fermentation; such is the case for Lactobacillus delbrueckii and S. thermophiles used in yogurt production. However, many bacteria perform heterolactic fermentation, producing a mixture of lactic acid, ethanol and/or acetic acid, and CO2 as a result, because of their use of the branched pentose phosphate pathway instead of the EMP pathway for glycolysis. One important heterolactic fermenter is Leuconostoc mesenteroides, which is used for souring vegetables like cucumbers and cabbage, producing pickles and sauerkraut, respectively.
Lactic acid bacteria are also important medically. The production of low pH environments within the body inhibits the establishment and growth of pathogens in these areas. For example, the vaginal microbiota is composed largely of lactic acid bacteria, but when these bacteria are reduced, yeast can proliferate, causing a yeast infection. Additionally, lactic acid bacteria are important in maintaining the health of the gastrointestinal tract and, as such, are the primary component of probiotics.
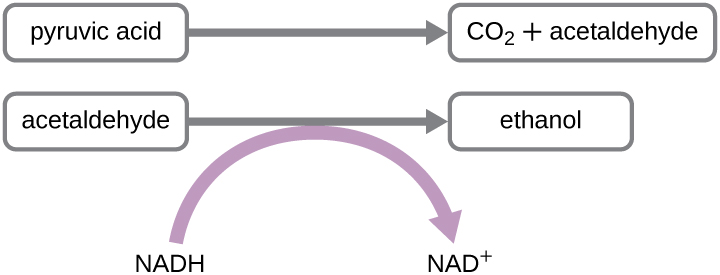
Another familiar fermentation process is alcohol fermentation by yeast, which produces ethanol. The ethanol fermentation reaction is shown in Figure \(\PageIndex{2}\). You may notice that unlike bacterial fermentations this fungal (eukaryotic) fermentation does not produce an acid as a waste product. The ethanol fermentation of pyruvate by the yeast Saccharomyces cerevisiae is used in the production of alcoholic beverages and also makes bread products rise due to CO2 production. Outside of the food industry, ethanol fermentation of plant products is important in biofuel production.
Beyond lactic acid fermentation and alcohol fermentation, many other fermentation methods occur in microbes, all for the purpose of ensuring an adequate supply of NAD+ for glycolysis (Table \(\PageIndex{2}\)). Without these pathways, glycolysis would not occur and no ATP would be harvested from the breakdown of glucose. It should be noted that most forms of fermentation besides homolactic fermentation produce gas, commonly CO2 and/or hydrogen gas. Many of these different types of fermentation pathways are also used in food production and each results in the production of different organic acids, contributing to the unique flavor of a particular fermented food product. The propionic acid produced during propionic acid fermentation contributes to the distinctive flavor of Swiss cheese, for example.
Several fermentation products are important commercially outside of the food industry. For example, chemical solvents such as acetone and butanol are produced during acetone-butanol-ethanol fermentation. Complex organic pharmaceutical compounds used in antibiotics (e.g., penicillin), vaccines, and vitamins are produced through mixed acid fermentation.
In addition to fermentation ability, fermentation products are used in the laboratory to differentiate various bacteria for diagnostic purposes. For example, enteric bacteria are known for their ability to perform mixed acid fermentation, reducing the pH, which can be detected using a pH indicator. Similarly, the bacterial production of acetoin during butanediol fermentation can also be detected. Gas production from fermentation can also be seen in an inverted Durham tube that traps produced gas in a broth culture.
| Pathway | End Products | Example Microbes | Commercial Products |
|---|---|---|---|
| Acetone-butanol-ethanol | Acetone, butanol, ethanol, CO2 | Clostridium acetobutylicum | Commercial solvents, gasoline alternative |
| Alcohol | Ethanol, CO2 | Candida, Saccharomyces | Beer, bread |
| Butanediol | Formic and lactic acid; ethanol; acetoin; 2,3 butanediol; CO2; hydrogen gas | Klebsiella, Enterobacter | Chardonnay wine |
| Butyric acid | Butyric acid, CO2, hydrogen gas | Clostridium butyricum | Butter |
| Lactic acid | Lactic acid | Streptococcus, Lactobacillus | Sauerkraut, yogurt, cheese |
| Mixed acid | Acetic, formic, lactic, and succinic acids; ethanol, CO2, hydrogen gas | Escherichia, Shigella | Vinegar, cosmetics, pharmaceuticals |
| Propionic acid | Acetic acid, propionic acid, CO2 | Propionibacterium, Bifidobacterium | Swiss cheese |
Exercise \(\PageIndex{1}\)
When would a metabolically versatile microbe perform fermentation rather than respiration?
IDENTIFYING BACTERIA BY USING API TEST PANELS
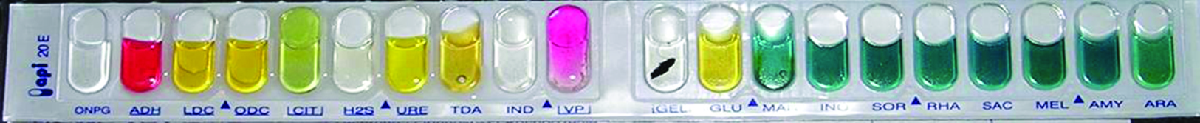
Identification of a microbial isolate is essential for the proper diagnosis and appropriate treatment of patients. Scientists have developed techniques that identify bacteria according to their biochemical characteristics. Typically, they either examine the use of specific carbon sources as substrates for fermentation or other metabolic reactions, or they identify fermentation products or specific enzymes present in reactions. In the past, microbiologists have used individual test tubes and plates to conduct biochemical testing. However, scientists, especially those in clinical laboratories, now more frequently use plastic, disposable, multitest panels that contain a number of miniature reaction tubes, each typically including a specific substrate and pH indicator. After inoculation of the test panel with a small sample of the microbe in question and incubation, scientists can compare the results to a database that includes the expected results for specific biochemical reactions for known microbes, thus enabling rapid identification of a sample microbe. These test panels have allowed scientists to reduce costs while improving efficiency and reproducibility by performing a larger number of tests simultaneously.
Many commercial, miniaturized biochemical test panels cover a number of clinically important groups of bacteria and yeasts. One of the earliest and most popular test panels is the Analytical Profile Index (API) panel invented in the 1970s. Once some basic laboratory characterization of a given strain has been performed, such as determining the strain’s Gram morphology, an appropriate test strip that contains 10 to 20 different biochemical tests for differentiating strains within that microbial group can be used. Currently, the various API strips can be used to quickly and easily identify more than 600 species of bacteria, both aerobic and anaerobic, and approximately 100 different types of yeasts. Based on the colors of the reactions when metabolic end products are present, due to the presence of pH indicators, a metabolic profile is created from the results (Figure \(\PageIndex{2}\)). Microbiologists can then compare the sample’s profile to the database to identify the specific microbe.
Key Concepts and Summary
- Fermentation uses an organic molecule as a final electron acceptor to regenerate NAD+ from NADH so that glycolysis can continue.
- Fermentation does not involve an electron transport system, and no ATP is made by the fermentation process directly. Fermenters make very little ATP—only two ATP molecules per glucose molecule during glycolysis.
- Microbial fermentation processes have been used for the production of foods and pharmaceuticals, and for the identification of microbes.
- During lactic acid fermentation, pyruvate accepts electrons from NADH and is reduced to lactic acid. Microbes performing homolactic fermentation produce only lactic acid as the fermentation product; microbes performing heterolactic fermentation produce a mixture of lactic acid, ethanol and/or acetic acid, and CO2.
- Lactic acid production by the normal microbiota prevents growth of pathogens in certain body regions and is important for the health of the gastrointestinal tract.
- During ethanol fermentation, pyruvate is first decarboxylated (releasing CO2) to acetaldehyde, which then accepts electrons from NADH, reducing acetaldehyde to ethanol. Ethanol fermentation is used for the production of alcoholic beverages, for making bread products rise, and for biofuel production.
- Fermentation products of pathways (e.g., propionic acid fermentation) provide distinctive flavors to food products. Fermentation is used to produce chemical solvents (acetone-butanol-ethanol fermentation) and pharmaceuticals (mixed acid fermentation).
- Specific types of microbes may be distinguished by their fermentation pathways and products. Microbes may also be differentiated according to the substrates they are able to ferment.
Contributors and Attributions
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


