2.5: Viral Structue and Prions
- Page ID
- 42717
- Describe the generalized structure of a viral particle including location and molecular make-up of genetic material, capsid, and envelope.
- Recognize and identify the most common capsid shapes.
- Provide two examples of the importance of protein folding and binding specificity in the replication of certain pathogens.
Key Points
- Viruses are classified into four groups based on type of genetic material and capsid shape.
- Viruses attach to their host cells to facilitate penetration of the cell membrane, allowing their replication inside the cell.
- Non-enveloped viruses can be more resistant to changes in temperature, pH, and some disinfectants than are enveloped viruses.
- The virus core contains the small single- or double-stranded genome that encodes the proteins that the virus cannot get from the host cell.
Viral Morphology
Viruses are acellular, meaning they are biological entities that do not have a cellular structure and they rely on a host cell for replication. Therefore, they lack most of the components of cells, such as organelles, ribosomes, and the plasma membrane. A virion (virus particle) consists of a nucleic acid core, an outer protein coating called a capsid, and sometimes an outer envelope made of phospholipid membranes derived from the host cell and embedded with viral proteins (Figure \(\PageIndex{1}\)). The capsid is made up of protein subunits called capsomeres. Viruses may also carry additional proteins, such as enzymes necessary for their replication (Figure \(\PageIndex{1}\)). The most obvious difference between members of viral families is their morphology, which is quite diverse. An interesting feature of viral complexity is that host and virion complexity are uncorrelated. Some of the most intricate virion structures are observed in bacteriophages, viruses that infect the simplest living organisms: bacteria.
Morphology
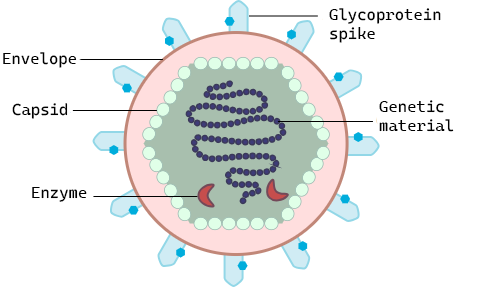
Figure \(\PageIndex{1}\): General virus structure: All viruses have genetic material (DNA or RNA) surrounded by a protein capsid and some also carry enzymes that will aid in their replication. They use glycoprotein spikes to attach to the host cell where they will replicate. The structure of the glycoprotein spikes determines to which cell surface proteins they can bind and therefore what host cells they can infect. In some viruses, the capsid is surrounded by a lipid envelope which is taken from the host cell when it is released.
Viruses of all shapes and sizes consist of a nucleic acid core, an outer protein coating or capsid, and sometimes an outer envelope. Viruses come in many shapes and sizes. In general, the shapes of viral capsid can be helical or icosahedral (Figure \(\PageIndex{2}\)). Viruses with a helical capsid and no envelope are often filamentous in shape. Filamentous viruses are long and cylindrical. Many plant viruses are filamentous, including TMV (tobacco mosaic virus). Icosahedral capsids are roughly spherical, such as poliovirus or herpesviruses. Enveloped viruses have membranes surrounding capsids (which can be either helical or icosahedral). Animal viruses, such as HIV, are frequently enveloped.
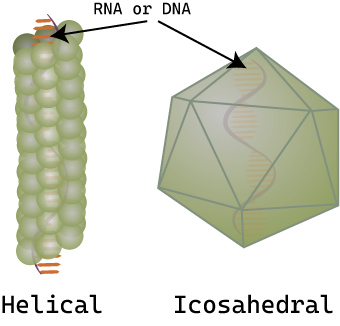
Figure \(\PageIndex{2}\): Examples of capsid shapes: Virus capsids are made of protein and have either a helical (tube-like) capsid or icosahedral (approximately spherical) capsid. The genetic material (DNA or RNA) is found inside the capsid.
Viruses use a specific glycoprotein (protein with attached sugars) to attach to their host cells via specific molecules which act as viral receptors (Figure \(\PageIndex{3}\)). The viral glycoproteins are often referred to as "spikes". These protein spikes must have a precise structure to be able to bind to the receptors on the host cell. Changes to the spike structure can lead to a change in the species or type of cell which the virus can infect. Attachment is a requirement for later penetration of the cell membrane, allowing them to complete their replication inside the cell. The receptors on the host cell that viruses use are molecules that are normally found on cell surfaces and have their own physiological functions. Viruses have simply evolved to make use of these molecules for their own replication.
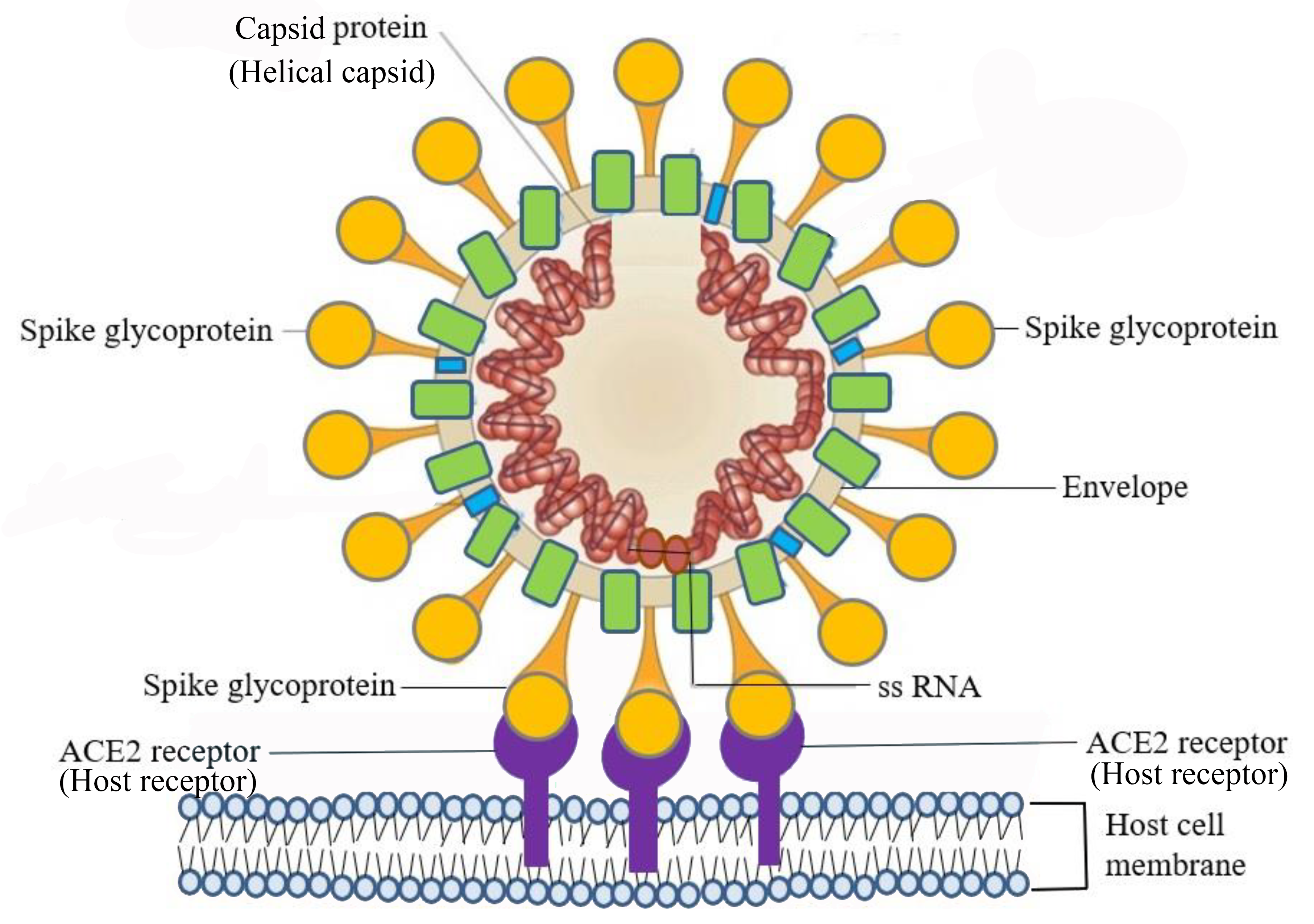
Figure \(\PageIndex{3}\): SARS–CoV-2 infects host cells through the specific binding of spike glycoprotein on the viral envelope and the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell’s surface. (CC BY 4.0 DEED; Adapted from Chen, S.-J.; Wang, S.-C.; Chen, Y.-C. Novel Antiviral Strategies in the Treatment of COVID-19: A Review. Microorganisms 2020, 8, 1259.)
Overall, the shape of the virion and the presence or absence of an envelope tell us little about what disease the virus may cause or what species it might infect, but they are still useful means to begin viral classification (Figure \(\PageIndex{4}\)). Among the most complex virions known, the T4 bacteriophage, which infects the Escherichia coli bacterium, has a tail structure that the virus uses to attach to host cells and a head structure that houses its DNA. Adenovirus, a non-enveloped animal virus that causes respiratory illnesses in humans, uses glycoprotein spikes protruding from its capsomeres to attach to host cells. Non-enveloped viruses also include those that cause polio (poliovirus), plantar warts (papillomavirus), and hepatitis A (hepatitis A virus).
Enveloped virions like HIV consist of nucleic acid and capsid proteins surrounded by a phospholipid bilayer envelope and its associated proteins. Glycoprotein spikes embedded in the viral envelope are used to attach to host cells. Other envelope proteins include the matrix proteins that stabilize the envelope and often play a role in the assembly of progeny virions. Chicken pox, influenza, and mumps are examples of diseases caused by viruses with envelopes. Because of the fragility of the envelope, non-enveloped viruses are more resistant to changes in temperature, pH, and some disinfectants than are enveloped viruses.
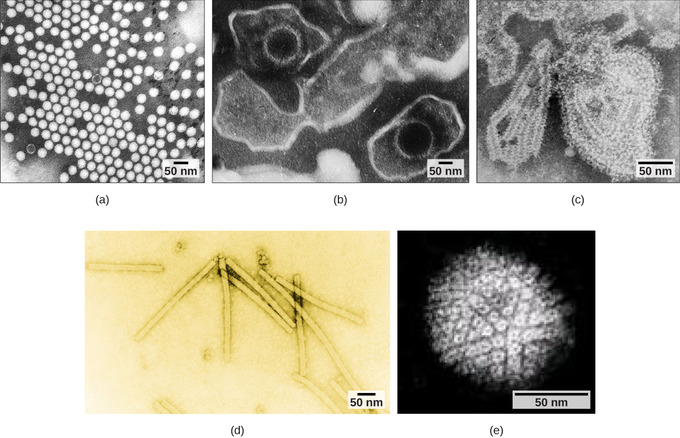
Figure \(\PageIndex{4}\): Adenovirus classification: Adenovirus (left) is depicted with a double-stranded DNA genome enclosed in an icosahedral capsid that is 90–100 nm across. The virus, shown clustered in the micrograph (right), is transmitted orally and causes a variety of illnesses in vertebrates, including human eye and respiratory infections. (Boundless General Biology)
Types of Nucleic Acid
Unlike nearly all living organisms that use DNA as their genetic material, viruses may use either DNA or RNA. The virus core contains the genome or total genetic content of the virus. Viral genomes tend to be small, containing only those genes that encode proteins that the virus cannot obtain from the host cell. This genetic material may be single- or double-stranded. It may also be linear or circular. While most viruses contain a single nucleic acid, others have genomes that have several, called segments.
In DNA viruses, the viral DNA directs the host cell’s replication proteins to synthesize new copies of the viral genome and to transcribe and translate that genome into viral proteins. DNA viruses cause human diseases, such as chicken pox, hepatitis B, and some venereal diseases, like herpes and genital warts.
RNA viruses contain only RNA as their genetic material. To replicate their genomes in the host cell, the RNA viruses encode enzymes that can replicate RNA into DNA, which cannot be done by the host cell. These RNA polymerase enzymes are more likely to make copying errors than DNA polymerases and, therefore, often make mistakes during transcription. For this reason, mutations in RNA viruses occur more frequently than in DNA viruses. This causes them to change and adapt more rapidly to their host. Human diseases caused by RNA viruses include hepatitis C, measles, and rabies.
Prions
- Contributed by OpenStax
- General Biology at OpenStax CNX
At one time, scientists believed that any infectious particle must contain DNA or RNA. Then, in 1982, Stanley Prusiner, a medical doctor studying scrapie (a fatal, degenerative disease in sheep) discovered that the disease was caused by proteinaceous infectious particles, or prions. Because proteins are acellular and do not contain DNA or RNA, Prusiner’s findings were originally met with resistance and skepticism; however, his research was eventually validated, and he received the Nobel Prize in Physiology or Medicine in 1997.
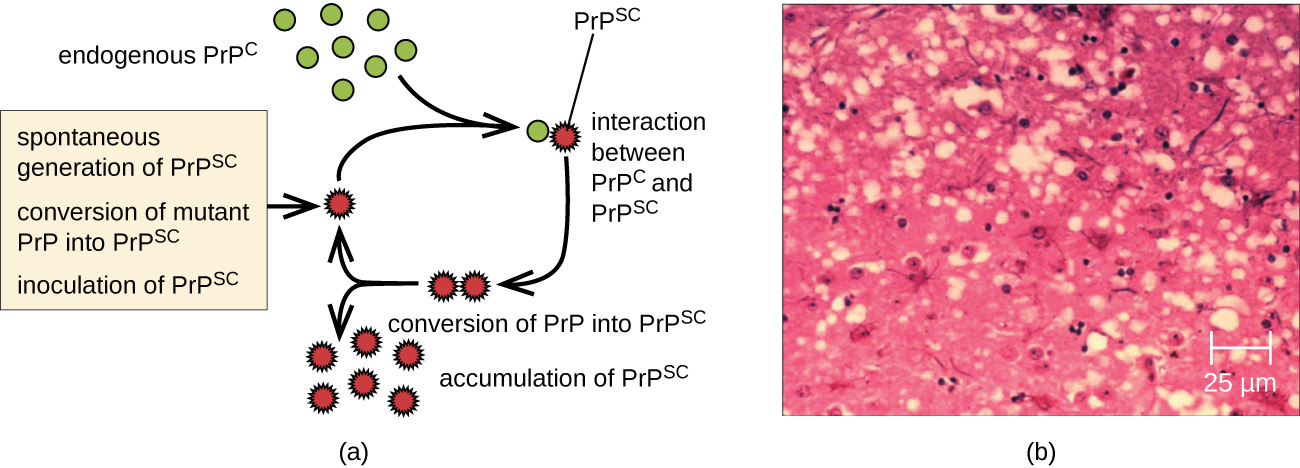
A prion is a misfolded rogue form of a normal protein (PrPc) found in the cell. This rogue prion protein (PrPsc), which may be caused by a genetic mutation or occur spontaneously, can be infectious, stimulating other endogenous normal proteins to become misfolded, forming plaques (see Figure \(\PageIndex{5}\)). The pathogenicity of prions relies entirely on the three-dimensional structure the protein assumes.
Today, prions are known to cause various forms of transmissible spongiform encephalopathy (TSE) in human and animals. TSE is a rare degenerative disorder that affects the brain and nervous system. The accumulation of rogue proteins causes the brain tissue to become sponge-like, killing brain cells and forming holes in the tissue, leading to brain damage, loss of motor coordination, and dementia (see Figure \(\PageIndex{6}\)). Infected individuals are mentally impaired and become unable to move or speak. There is no cure, and the disease progresses rapidly, eventually leading to death within a few months or years.
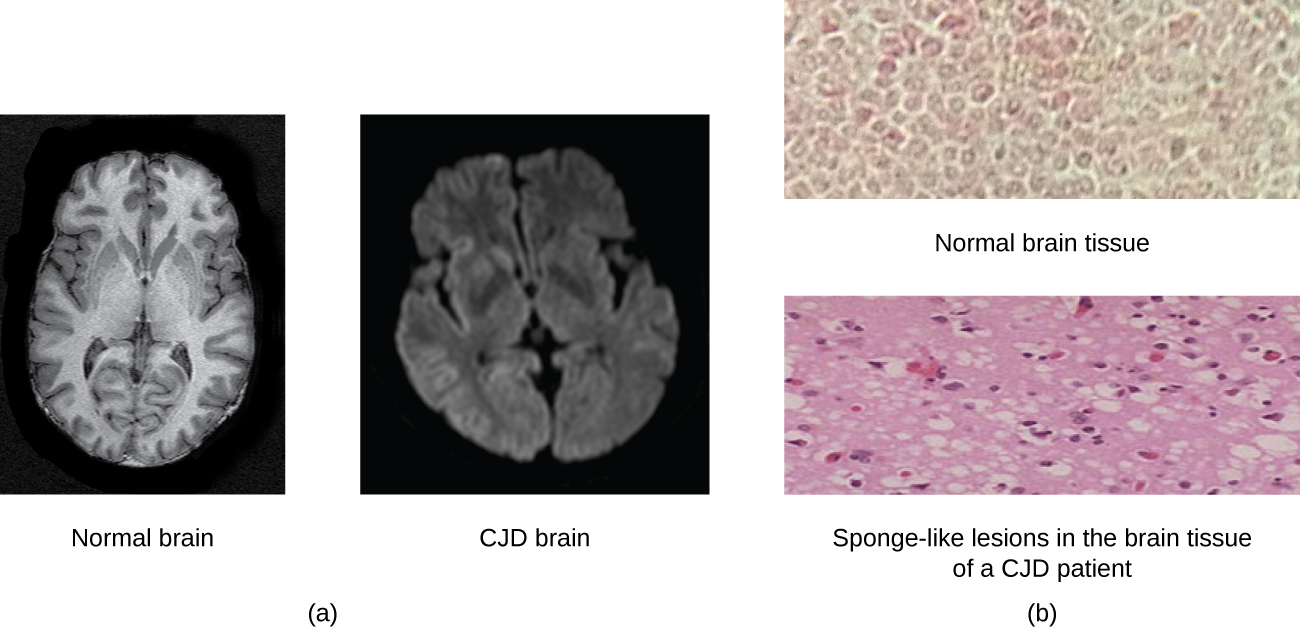
TSEs in humans include kuru, fatal familial insomnia, Gerstmann-Straussler-Scheinker disease, and Creutzfeldt-Jakob disease (see Figure \(\PageIndex{6}\)). TSEs in animals include mad cow disease, scrapie (in sheep and goats), and chronic wasting disease (in elk and deer). TSEs can be transmitted between animals and from animals to humans by eating contaminated meat or animal feed. Transmission between humans can occur through heredity (as is often the case with GSS and CJD) or by contact with contaminated tissue, as might occur during a blood transfusion or organ transplant. There is no evidence for transmission via casual contact with an infected person. Table \(\PageIndex{1}\) lists TSEs that affect humans and their modes of transmission.
| Disease | Mechanism(s) of Transmission1 |
|---|---|
| Sporadic CJD (sCJD) | Not known; possibly by alteration of normal prior protein (PrP) to rogue form due to somatic mutation |
| Variant CJD (vCJD) | Eating contaminated cattle products and by secondary bloodborne transmission |
| Familial CJD (fCJD) | Mutation in germline PrP gene |
| Iatrogenic CJD (iCJD) | Contaminated neurosurgical instruments, corneal graft, gonadotrophic hormone, and, secondarily, by blood transfusion |
| Kuru | Eating infected meat through ritualistic cannibalism |
| Gerstmann-Straussler-Scheinker disease (GSS) | Mutation in germline PrP gene |
| Fatal familial insomnia (FFI) | Mutation in germline PrP gene |
Prions are extremely difficult to destroy because they are resistant to heat, chemicals, and radiation. Even standard sterilization procedures do not ensure the destruction of these particles. Currently, there is no treatment or cure for TSE disease, and contaminated meats or infected animals must be handled according to federal guidelines to prevent transmission.


