11: Bacterial Communities
- Page ID
- 42550
Chapter 11 BSC 3271 Learning Objectives
- Describe the structure of biofilms, including the shape of mature biofilm and the role of exopolysaccharide.
- Describe how exopolysaccharide protects the biolofilm against environmental stress (including disinfectants and antibiotics), helps cells in the biofilm acquire nutrients, and evade the immune system
- Describe the steps of biofilm formation and development.
- Explain how bacteria in a biofilm differ from planktonic cells in cell structure and metabolically.
- Recognize common sites of biofilm formation in the body and how the biofilm lifestyle can lead to systemic infection.
- Explain the mechanism of quorum sensing and the effect of quorum sensing on cells (in general)
- Explain (generally) how expression of the genes for coagulase and kinase is affected by quorum sensing in Staph. aureus.
- Describe the general cellular, metabolic, and physiological characteristics of Pseudomonas (also see Chapter 15)
- Identify the most common infections caused by P. aeruginosa and which populations are most at risk for infection (also see Chapter 15)
- Explain the link between biofilm formation, quorum sensing, and the ability to cause disease in P.aeruginosa
- Explain the link between quorum sensing and genetic exchange, particularly in Bacillus sp. and Enterococcus (VRE).
Biofilms
In nature, microorganisms grow mainly in biofilms, complex and dynamic ecosystems that form on a variety of environmental surfaces, from industrial conduits and water treatment pipelines to rocks in river beds. The bacteria found in mature biofilms are much less metabolically active than free-living planktonic bacteria and resemble bacteria in stationary phase of the growth curve rather than log phase. Biofilms are not restricted to solid surface substrates, however. Almost any surface in a liquid environment containing some minimal nutrients will eventually develop a biofilm. Microbial mats that float on water, for example, are biofilms that contain large populations of photosynthetic microorganisms. Biofilms found in the human mouth may contain hundreds of bacterial species. Regardless of the environment where they occur, biofilms are not random collections of microorganisms; rather, they are highly structured communities that provide a selective advantage to their constituent microorganisms.
Biofilm Structure
Observations using confocal microscopy have shown that environmental conditions influence the overall structure of biofilms. Filamentous biofilms called streamers form in rapidly flowing water, such as freshwater streams, eddies, and specially designed laboratory flow cells that replicate growth conditions in fast-moving fluids. The streamers are anchored to the substrate by a “head” and the “tail” floats downstream in the current. In still or slow-moving water, biofilms mainly assume a mushroom-like shape. The structure of biofilms may also change with other environmental conditions such as nutrient availability.
Detailed observations of biofilms under confocal laser and scanning electron microscopes reveal clusters of microorganisms embedded in a matrix interspersed with open water channels. The extracellular matrix consists of extracellular polymeric substances (EPS) secreted by the organisms in the biofilm. The extracellular matrix represents a large fraction of the biofilm, accounting for 50%–90% of the total dry mass. The properties of the EPS vary according to the resident organisms and environmental conditions.
EPS is a hydrated gel composed primarily of polysaccharides and containing other macromolecules such as proteins, nucleic acids, and lipids. It plays a key role in maintaining the integrity and function of the biofilm. Channels in the EPS allow movement of nutrients, waste, and gases throughout the biofilm. This keeps the cells hydrated, preventing desiccation. EPS also shelters organisms in the biofilm from predation by other microbes or cells (e.g., protozoans, white blood cells in the human body).
Biofilm Formation
Free-floating microbial cells that live in an aquatic environment are called planktonic cells. The formation of a biofilm essentially involves the attachment of planktonic cells to a substrate, where they become sessile (attached to a surface). This occurs in stages, as depicted in Figure \(\PageIndex{1}\). The first stage involves the attachment of planktonic cells to a surface coated with a conditioning film of organic material. At this point, attachment to the substrate is reversible, but as cells express new phenotypes that facilitate the formation of EPS, they transition from a planktonic to a sessile lifestyle. The biofilm develops characteristic structures, including an extensive matrix and water channels. Appendages such as fimbriae, pili, and flagella interact with the EPS, and microscopy and genetic analysis suggest that such structures are required for the establishment of a mature biofilm. In the last stage of the biofilm life cycle, cells on the interior of the biofilm become stressed from lack of nutrients and revert to a planktonic lifestyle, sloughing off the mature biofilm to colonize new sites. This stage is referred to as dispersal.
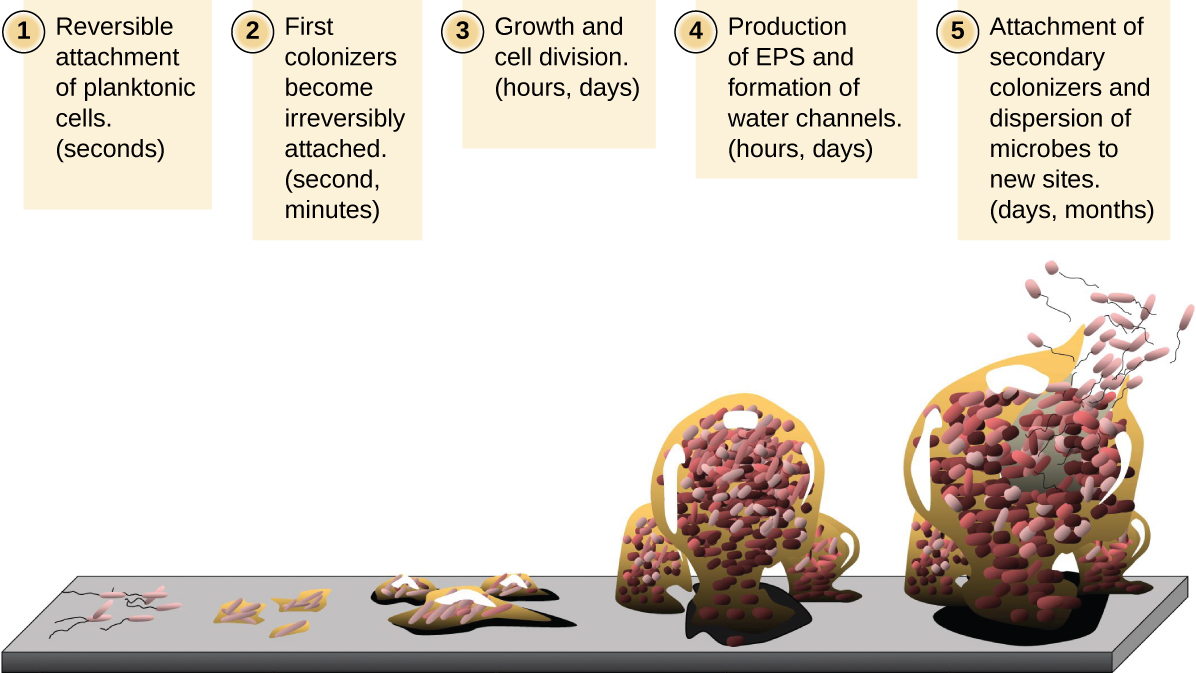
Within a biofilm, different species of microorganisms establish metabolic collaborations in which the waste product of one organism becomes the nutrient for another. For example, aerobic microorganisms consume oxygen, creating anaerobic regions that promote the growth of anaerobes. This occurs in many polymicrobial infections that involve both aerobic and anaerobic pathogens.
Biofilms and Human Health
The human body harbors many types of biofilms, some beneficial and some harmful. For example, the layers of normal microbiota lining the intestinal and respiratory mucosa play a role in warding off infections by pathogens. However, other biofilms in the body can have a detrimental effect on health. For example, the plaque that forms on teeth is a biofilm that can contribute to dental and periodontal disease. Biofilms can also form in wounds, sometimes causing serious infections that can spread. The bacterium Pseudomonas aeruginosa often colonizes biofilms in the airways of patients with cystic fibrosis, causing chronic and sometimes fatal infections of the lungs. Biofilms can also form on medical devices used in or on the body, causing infections in patients with in-dwelling catheters, artificial joints, or contact lenses.
Pathogens embedded within biofilms exhibit a higher resistance to antibiotics than their free-floating counterparts. Several hypotheses have been proposed to explain why. Cells in the deep layers of a biofilm are metabolically inactive and may be less susceptible to the action of antibiotics that disrupt metabolic activities. The EPS may also slow the diffusion of antibiotics and antiseptics, preventing them from reaching cells in the deeper layers of the biofilm. Phenotypic changes may also contribute to the increased resistance exhibited by bacterial cells in biofilms. For example, the increased production of efflux pumps, membrane-embedded proteins that actively extrude antibiotics out of bacterial cells, have been shown to be an important mechanism of antibiotic resistance among biofilm-associated bacteria. Finally, biofilms provide an ideal environment for horizontal gene transfer (HGT), which often includes genes that confer antibiotic resistance.
Biofilms, Persisters, and Antibiotic Tolerance
(adapted from Boundless)
The EPS produced by biofilms protects them from external threats, such as attacks by the body’s immune cells. The property of biofilms constitute a penetration barrier for most antibiotics therefore preventing the drug from reaching the microbes. It is being widely recognized that bacterial biofilms are responsible for several chronic diseases that are difficult to treat, hence hard to eradicate (e.g., cystitis, endocarditis, urinary tract infections, gingivitis, dental plaque, and other yet to be identified conditions). They differ from free-floating or planktonic bacteria that cause acute infections and are managed by antimicrobial drugs.

Persisters are multidrug tolerant cells present in all bacterial populations. Bacterial populations that produce persister cells that neither grow nor die in the presence of microbicidal antibiotics are largely responsible for high levels of biofilm tolerance to antimicrobials. Persisters are not mutants, but rather phenotypic variants of the wild-type that upon inoculation produce a culture with similar levels of tolerance. Elimination of persisters remains an obstacle for the eradication of some tenacious and highly recurrent bacterial infections. Biofilms and persisters are the cause of multidrug tolerance. Multidrug tolerance differs from multidrug resistance in that it is not caused by mutant microbes but rather by microbial cells that exist in a transient, dormant state. These non-dividing cells often survive antibiotic exposure targeted to kill highly proliferating bacteria.
Quorum Sensing
Quorum Sensing Mechanism
The mechanism by which cells in a biofilm coordinate their activities in response to environmental stimuli is called quorum sensing. Quorum sensing—which can occur between cells of different species within a biofilm—enables microorganisms to detect their cell density through the release and binding of small, diffusible molecules called autoinducers. Each individual cell always produces a small amount of autoinducer (Figure \(\PageIndex{3A}\)). The concentration of autoinducer in the environment increases as the population size increases. When the cell population reaches a critical threshold (a quorum), these autoinducers initiate a cascade of reactions that activate genes associated with cellular functions that are beneficial only when the population reaches a critical density (Figure \(\PageIndex{3B}\)). For example, in some pathogens, synthesis of virulence factors only begins when enough cells are present to overwhelm the immune defenses of the host. Although mostly studied in bacterial populations, quorum sensing takes place between bacteria and eukaryotes and between eukaryotic cells such as the fungus Candida albicans, a common member of the human microbiota that can cause infections in immunocompromised individuals.
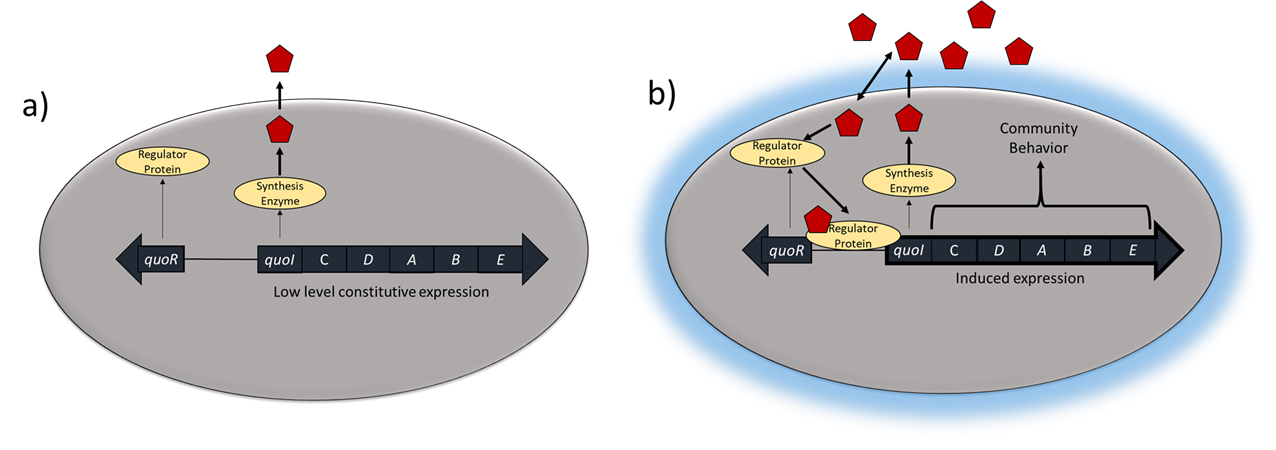
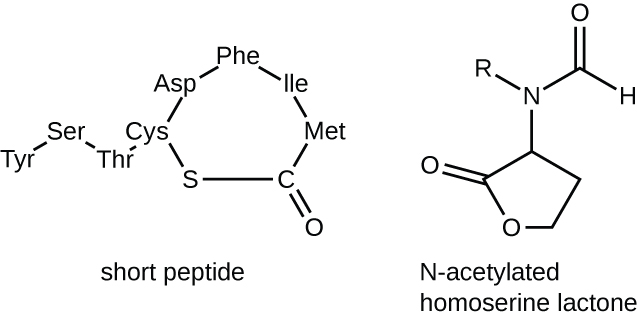
The signaling molecules in quorum sensing belong to two major classes. Gram-negative bacteria communicate mainly using N-acylated homoserine lactones, whereas gram-positive bacteria mostly use small peptides (Figure \(\PageIndex{4}\)). In all cases, the first step in quorum sensing consists of the binding of the autoinducer to its specific receptor only when a threshold concentration of signaling molecules is reached. Once binding to the receptor takes place, a cascade of signaling events leads to changes in gene expression. The result is the activation of biological responses linked to quorum sensing, notably an increase in the production of signaling molecules themselves, hence the term autoinducer.
Virulence and Quorum Sensing
Many organisms regulate genes for various virulence factors using quorum sensing. In many cases, it is a waste of energy for a bacterium to express genes for virulence factors at low population size because it would have effect on the host. When the population size is large enough, however, (and the bacteria are in a host) producing virulence factors allows the community of bacteria to successfully invade the host. For example, biofilm formation and toxin production (both of which are required to cause infection) are controlled by quorum sensing in the opportunistic pathogen Pseudomonas aeruginosa.
Another good example is control of the expression of two enzymes in Staphylococcus aureus with seemingly cantradictory effects. Coagulase (which is neccessary for virulence in S. aures) causes the polymerization of the protein fibrin whereas kinase (sometimes called staphylokinase) results in its depolymerization (breakdown). At low population levels, S. aureus produces coagulase. The polymerized fibrin coats the cells of S. aureus like a capsule and hides the cells from the immune system. Once the population size is large enough, however, coagulase production is turned off and kinase is turned on. The fibrin is depolymerized and the S. aureus is released to invade the body further.
As antibiotic resistance increases, inhibiting quorum sensing has been extensively studied as a way to control infections as an alternative to antibiotics.
Genetic Exchange and Quorum Sensing
Horizontal gene transfer (HGT) requires the presence of other compatible organisms, so it is not surprising that all three forms of HGT can be controlled by quorum sensing. For instance, competence (along with endospore and antibiotic production) in Bacillus is regulated by quorum sensing. Many phages will shift from lysogenic to lytic in response to quorum sensing signals, resulting in increased transduction. Quorum sensing has been directly linked to the spread of vancomycin resistance in vancomycin-resistant Enterococci (VRE). Quorum sensing signals are used in Enterococcus to determine when (and with which bacteria) to go through conjugation. Plasmids which carry vancomycin resistance genes have been shown to be regulated by quorum sensing. Understanding quorum sensing, therefore, can help us develop novel methods of controlling the spread of antibiotic resistance.
Exercise \(\PageIndex{9}\)
- What is the matrix of a biofilm composed of?
- What is the role of quorum sensing in a biofilm?
Thumbnail: "Biofilm de kombucha" by Charles de Mille-Isles is licensed under CC BY 2.0

