6.6: Anabolism
- Page ID
- 46683
Anabolic Pathways
(The following material is adapted from Kaiser Microbiology)
Anabolism, often called biosynthesis, is the metabolic production of molecules used in the structure and function of the cell from simpler organic molecules. Often the starting substrates for anabolic pathways are intermediates of Central Metabolism referred to as precursor metabolites. Just like constructing a building requires energy input and nails in addition to building materials, anabolism also requires input of ATP and electrons (usually in the form of NADPH as opposed to NADH) in addition to precursor metabolites. Some anabolic pathways also require additional building materials such as nitrogen, phosphate, or sulfur.
Catabolic pathways provide the energy that fuel anabolic pathways. Another factor that links catabolic and anabolic pathways is the generation of precursor metabolites. Precursor metabolites are intermediate molecules in Central Metabolism that can be either oxidized to generate ATP or can be used to synthesize macromolecular subunits such as amino acids, lipids, and nucleotides as shown in Figure \(\PageIndex{1}\)
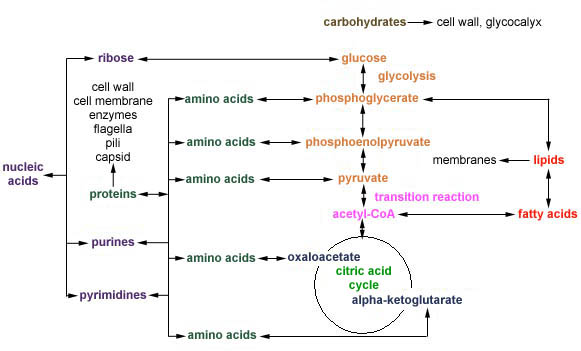
Amino Acid Biosynthesis
(This section is adapted from General Microbiology at Boundless)
Amino acids are the structural units that make up proteins. They join together to form short polymer chains called peptides or longer chains called either polypeptides or proteins. These polymers are linear and unbranched, with each amino acid within the chain attached to two neighboring amino acids. The process of making proteins is called translation and involves the step-by-step addition of amino acids to a growing protein chain by a ribozyme that is called a ribosome. The order in which the amino acids are added is read through the genetic code from an mRNA template, which is a RNA copy of one of the organism ‘s genes.
Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all 20. Some simple parasites, such as the bacteria Mycoplasma pneumoniae, lack all amino acid synthesis and take their amino acids directly from their hosts. All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. In most amino acid anabolic pathways, nitrogen is provided by transamination using the amino acids glutamate or glutamine (Figure \(\PageIndex{2}\)). Amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated using glutamate or glutamine to form an amino acid.
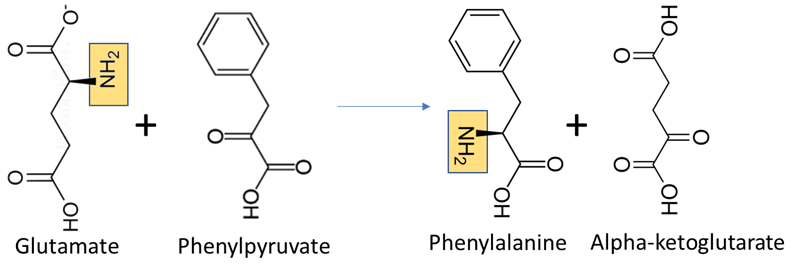
Glutamate and glutamine, on the other hand, can be formed by direct addition of ammonium to alpha-ketoglutarate or glutamate to form glutamate or glutamine, respectively. This process is called amination (Figure \(\PageIndex{3}\). The amination of alpha-ketoglutarate to form glutamate is how bacteria transform inorganic nitrogen to organic nitrogen, assimilating it into the cell.

Nitrogen Assimilation
(This section is adapted from content contributed by Linda Bruslind)
Assimilation
Assimilation is a reductive process by which an inorganic form of nitrogen is reduced to organic nitrogen compounds such as amino acids and nucleotides, allowing for cellular growth and reproduction. Only the amount needed by the cell is reduced. Ammonia assimilation occurs when the ammonia (NH3)/ammonium ion (NH4+) formed during nitrogen fixation is used to aminate alpha-ketoglutarate to form glutamate. Assimilative nitrate reduction is a reduction of nitrate to cellular nitrogen, in a multi-step process where nitrate is reduced to nitrite then ammonia and finally into organic nitrogen.
Nitrogen Fixation
Nitrogen fixation describes the conversion of the relatively inert dinitrogen gas (N2) into ammonia (NH3), a much more useable form of nitrogen for most life forms. The process is performed by diazotrophs, a limited number of bacteria and archaea that can grow without an external source of fixed nitrogen, because of their abilities. Nitrogen fixation is an essential process for Earth’s organisms, since nitrogen is a required component of various organic molecules, such as amino acids and nucleotides. Plants, animals, and other organisms rely on bacteria and archaea to provide nitrogen in a fixed form, since no eukaryote is known that can fix nitrogen.
Nitrogen fixation is an extremely energy and electron intensive process, in order to break the triple bond in N2 and reduce it to NH3. It requires a particular enzyme known as nitrogenase, which is inactivated by O2. Thus, nitrogen fixation must take place in an anaerobic environment. Aerobic nitrogen-fixing organisms must devise special conditions or arrangements in order to protect their enzyme. Nitrogen-fixing organisms can either exist independently or pair up with a plant host:
- Symbiotic nitrogen-fixing organisms: these bacteria partner up with a plant, to provide them with an environment appropriate for the functioning of their nitrogenase enzyme. The bacteria live in the plant’s tissue, often in root nodules, fixing nitrogen and sharing the results. The plant provides both the location to fix nitrogen, as well as additional nutrients to support the energy-taxing process of nitrogen fixation. It has been shown that the bacteria and the host exchange chemical recognition signals that facilitate the relationship. One of the best known bacteria in this category is Rhizobium, which partners up with plants of the legume family (clover, soybeans, alfalfa, etc). Legumes are well known for their high protein content, and their partnership with the nitrogen-fixing Rhizobium contributes to their ability to make large quantities of these nitrogen-rich compounds.
- Free-living nitrogen-fixing organisms: these organisms, both bacteria and archaea, fix nitrogen for their own use that ends up being shared when the organisms dies or is ingested. Free-living nitrogen-fixing organisms that grow anaerobically do not have to worry about special adaptations for their nitrogenase enzyme. Aerobic organisms must make adaptations. Cyanobacteria, a multicellular bacterium, make specialized cells known as heterocystsin which nitrogen fixation occurs. Since Cyanobacteria produce oxygen as part of their photosynthesis, an anoxygenic version occurs within the heterocyst, allowing the nitrogenase to remain active. The heterocysts share the fixed nitrogen with surrounding cells, while the surrounding cells provide additional nutrients to the heterocysts.
Key Points
- Anabolic pathways rely on the energy and precursor metabolites formed through catabolism.
- All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway.
- Amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated to form an amino acid.
- Most bacteria assimilate nitrogen as ammonium, which is used to aminate alpha-ketoglutarate (from the TCA cycle) to make glutamate. Some can convert nitrate to ammonium which is then assimilated.
- Only a few prokaryotes have the ability to assimilate atmospheric nitrogen in a process called nitrogen fixation. Two notable examples are the legume symbionts Rhizobium and the cyanobacteria.

