8.3: The Effects of pH and Temperature on Microbial Growth
- Page ID
- 31819
Learning Objectives
- Illustrate and briefly describe minimum, optimum, and maximum pH requirements for growth
- Identify and describe the different categories of microbes with pH requirements for growth: acidophiles, neutrophiles, and alkaliphiles
- Illustrate and briefly describe minimum, optimum, and maximum temperature requirements for growth
- Identify and describe different categories of microbes with temperature requirements for growth: psychrophile, psychrotrophs, mesophile, thermophile, hyperthermophile
Effects of pH
Yogurt, pickles, sauerkraut, and lime-seasoned dishes all owe their tangy taste to a high acid content (Figure \(\PageIndex{1}\)). Recall that acidity is a function of the concentration of hydrogen ions [H+] and is measured as pH. Environments with pH values below 7.0 are considered acidic, whereas those with pH values above 7.0 are considered basic. Extreme pH affects the structure of all macromolecules. The hydrogen bonds holding together strands of DNA break up at high pH. Lipids are hydrolyzed by an extremely basic pH. The proton motive force responsible for production of ATP in cellular respiration depends on the concentration gradient of H+ across the plasma membrane. If H+ ions are neutralized by hydroxide ions, the concentration gradient collapses and impairs energy production. But the component most sensitive to pH in the cell is its workhorse, the protein. Moderate changes in pH modify the ionization of amino-acid functional groups and disrupt hydrogen bonding, which, in turn, promotes changes in the folding of the molecule, promoting denaturation and destroying activity.

The optimum growth pH is the most favorable pH for the growth of an organism. The lowest pH value that an organism can tolerate is called the minimum growth pH and the highest pH is the maximum growth pH. These values can cover a wide range, which is important for the preservation of food and to microorganisms’ survival in the stomach. For example, the optimum growth pH of Salmonella spp. is 7.0–7.5, but the minimum growth pH is closer to 4.2.
Most bacteria are neutrophiles, meaning they grow optimally at a pH within one or two pH units of the neutral pH of 7 (see Figure \(\PageIndex{2}\)). Most familiar bacteria, like Escherichia coli, staphylococci, and Salmonella spp. are neutrophiles and do not fare well in the acidic pH of the stomach. However, there are pathogenic strains of E. coli, S. typhi, and other species of intestinal pathogens that are much more resistant to stomach acid. In comparison, fungi thrive at slightly acidic pH values of 5.0–6.0.
Microorganisms that grow optimally at pH less than 5.55 are called acidophiles. For example, the sulfur-oxidizing Sulfolobus spp. isolated from sulfur mud fields and hot springs in Yellowstone National Park are extreme acidophiles. These archaea survive at pH values of 2.5–3.5. Species of the archaean genus Ferroplasma live in acid mine drainage at pH values of 0–2.9. Lactobacillus bacteria, which are an important part of the normal microbiota of the vagina, can tolerate acidic environments at pH values 3.5–6.8 and also contribute to the acidity of the vagina (pH of 4, except at the onset of menstruation) through their metabolic production of lactic acid. The vagina’s acidity plays an important role in inhibiting other microbes that are less tolerant of acidity. Acidophilic microorganisms display a number of adaptations to survive in strong acidic environments. For example, proteins show increased negative surface charge that stabilizes them at low pH. Pumps actively eject H+ ions out of the cells. The changes in the composition of membrane phospholipids probably reflect the need to maintain membrane fluidity at low pH.
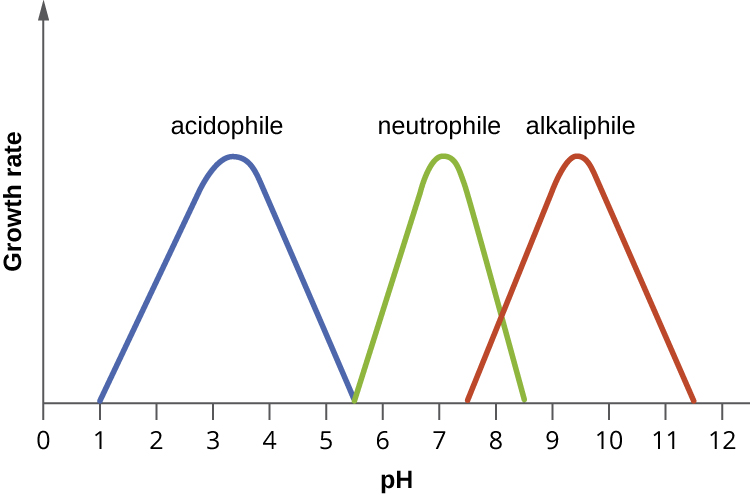
At the other end of the spectrum are alkaliphiles, microorganisms that grow best at pH between 8.0 and 10.5. Vibrio cholerae, the pathogenic agent of cholera, grows best at the slightly basic pH of 8.0; it can survive pH values of 11.0 but is inactivated by the acid of the stomach. When it comes to survival at high pH, the bright pink archaean Natronobacterium, found in the soda lakes of the African Rift Valley, may hold the record at a pH of 10.5 (Figure \(\PageIndex{3}\)). Extreme alkaliphiles have adapted to their harsh environment through evolutionary modification of lipid and protein structure and compensatory mechanisms to maintain the proton motive force in an alkaline environment. For example, the alkaliphile Bacillus firmus derives the energy for transport reactions and motility from a Na+ ion gradient rather than a proton motive force. Many enzymes from alkaliphiles have a higher isoelectric point, due to an increase in the number of basic amino acids, than homologous enzymes from neutrophiles.

Survival at the Low pH of the Stomach
Peptic ulcers (or stomach ulcers) are painful sores on the stomach lining. Until the 1980s, they were believed to be caused by spicy foods, stress, or a combination of both. Patients were typically advised to eat bland foods, take anti-acid medications, and avoid stress. These remedies were not particularly effective, and the condition often recurred. This all changed dramatically when the real cause of most peptic ulcers was discovered to be a slim, corkscrew-shaped bacterium, Helicobacter pylori. This organism was identified and isolated by Barry Marshall and Robin Warren, whose discovery earned them the Nobel Prize in Medicine in 2005.
The ability of H. pylori to survive the low pH of the stomach would seem to suggest that it is an extreme acidophile. As it turns out, this is not the case. In fact, H. pylori is a neutrophile. So, how does it survive in the stomach? Remarkably, H. pylori creates a microenvironment in which the pH is nearly neutral. It achieves this by producing large amounts of the enzyme urease, which breaks down urea to form NH4+ and CO2. The ammonium ion raises the pH of the immediate environment.
This metabolic capability of H. pylori is the basis of an accurate, noninvasive test for infection. The patient is given a solution of urea containing radioactively labeled carbon atoms. If H. pylori is present in the stomach, it will rapidly break down the urea, producing radioactive CO2 that can be detected in the patient’s breath. Because peptic ulcers may lead to gastric cancer, patients who are determined to have H. pylori infections are treated with antibiotics.
Exercise \(\PageIndex{1}\)
- What effect do extremes of pH have on proteins?
- What pH-adaptive type of bacteria would most human pathogens be?
Effects of Temperature
When the exploration of Lake Whillans started in Antarctica, researchers did not expect to find much life. Constant subzero temperatures and lack of obvious sources of nutrients did not seem to be conditions that would support a thriving ecosystem. To their surprise, the samples retrieved from the lake showed abundant microbial life. In a different but equally harsh setting, bacteria grow at the bottom of the ocean in sea vents (Figure \(\PageIndex{1}\)), where temperatures can reach 340 °C (700 °F).
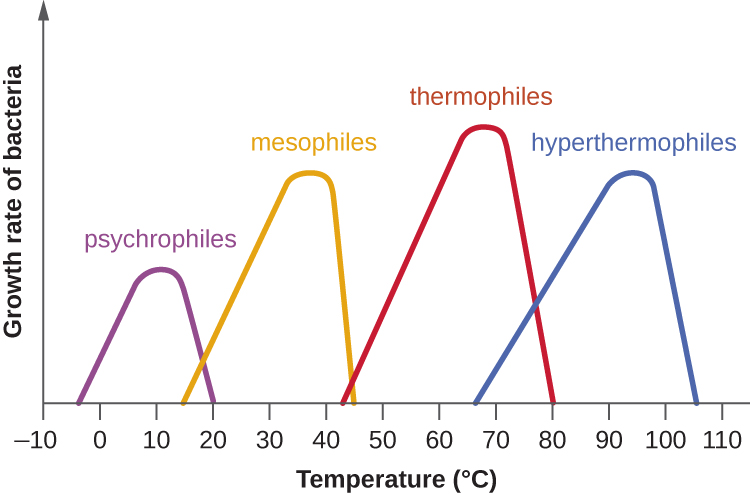
Microbes can be roughly classified according to the range of temperature at which they can grow. The growth rates are the highest at the optimum growth temperature for the organism. The lowest temperature at which the organism can survive and replicate is its minimum growth temperature. The highest temperature at which growth can occur is its maximum growth temperature. The following ranges of permissive growth temperatures are approximate only and can vary according to other environmental factors.
Organisms categorized as mesophiles (“middle loving”) are adapted to moderate temperatures, with optimal growth temperatures ranging from room temperature (about 20 °C) to about 45 °C. As would be expected from the core temperature of the human body, 37 °C (98.6 °F), normal human microbiota and pathogens (e.g., E. coli, Salmonella spp., and Lactobacillus spp.) are mesophiles.
Organisms called psychrotrophs, also known as psychrotolerant, prefer cooler environments, from a high temperature of 25 °C to refrigeration temperature about 4 °C. They are found in many natural environments in temperate climates. They are also responsible for the spoilage of refrigerated food.
Clinical Focus- Resolution
The presence of Listeria in Jeni’s blood suggests that her symptoms are due to listeriosis, an infection caused by L. monocytogenes. Listeriosis is a serious infection with a 20% mortality rate and is a particular risk to Jeni’s fetus. A sample from the amniotic fluid cultured for the presence of Listeria gave negative results. Because the absence of organisms does not rule out the possibility of infection, a molecular test based on the nucleic acid amplification of the 16S ribosomal RNA of Listeria was performed to confirm that no bacteria crossed the placenta. Fortunately, the results from the molecular test were also negative.
Jeni was admitted to the hospital for treatment and recovery. She received a high dose of two antibiotics intravenously for 2 weeks. The preferred drugs for the treatment of listeriosis are ampicillin or penicillin G with an aminoglycoside antibiotic. Resistance to common antibiotics is still rare in Listeria and antibiotic treatment is usually successful. She was released to home care after a week and fully recovered from her infection.
L. monocytogenes is a gram-positive short rod found in soil, water, and food. It is classified as a psychrophile and is halotolerant. Its ability to multiply at refrigeration temperatures (4–10 °C) and its tolerance for high concentrations of salt (up to 10% sodium chloride [NaCl]) make it a frequent source of food poisoning. Because Listeria can infect animals, it often contaminates food such as meat, fish, or dairy products. Contamination of commercial foods can often be traced to persistent biofilms that form on manufacturing equipment that is not sufficiently cleaned.
Listeria infection is relatively common among pregnant women because the elevated levels of progesterone downregulate the immune system, making them more vulnerable to infection. The pathogen can cross the placenta and infect the fetus, often resulting in miscarriage, stillbirth, or fatal neonatal infection. Pregnant women are thus advised to avoid consumption of soft cheeses, refrigerated cold cuts, smoked seafood, and unpasteurized dairy products. Because Listeria bacteria can easily be confused with diphtheroids, another common group of gram-positive rods, it is important to alert the laboratory when listeriosis is suspected.
The organisms retrieved from arctic lakes such as Lake Whillans are considered extreme psychrophiles (cold loving). Psychrophiles are microorganisms that can grow at 0 °C and below, have an optimum growth temperature close to 15 °C, and usually do not survive at temperatures above 20 °C. They are found in permanently cold environments such as the deep waters of the oceans. Because they are active at low temperature, psychrophiles and psychrotrophs are important decomposers in cold climates.

Organisms that grow at optimum temperatures of 50 °C to a maximum of 80 °C are called thermophiles (“heat loving”). They do not multiply at room temperature. Thermophiles are widely distributed in hot springs, geothermal soils, and manmade environments such as garden compost piles where the microbes break down kitchen scraps and vegetal material. Examples of thermophiles include Thermus aquaticus and Geobacillus spp. Higher up on the extreme temperature scale we find the hyperthermophiles, which are characterized by growth ranges from 80 °C to a maximum of 110 °C, with some extreme examples that survive temperatures above 121 °C, the average temperature of an autoclave. The hydrothermal vents at the bottom of the ocean are a prime example of extreme environments, with temperatures reaching an estimated 340 °C (Figure \(\PageIndex{4}\)). Microbes isolated from the vents achieve optimal growth at temperatures higher than 100 °C. Noteworthy examples are Pyrobolus and Pyrodictium, archaea that grow at 105 °C and survive autoclaving. Figure \(\PageIndex{5}\) shows the typical skewed curves of temperature-dependent growth for the categories of microorganisms we have discussed.
Life in extreme environments raises fascinating questions about the adaptation of macromolecules and metabolic processes. Very low temperatures affect cells in many ways. Membranes lose their fluidity and are damaged by ice crystal formation. Chemical reactions and diffusion slow considerably. Proteins become too rigid to catalyze reactions and may undergo denaturation. At the opposite end of the temperature spectrum, heat denatures proteins and nucleic acids. Increased fluidity impairs metabolic processes in membranes. Some of the practical applications of the destructive effects of heat on microbes are sterilization by steam, pasteurization, and incineration of inoculating loops. Proteins in psychrophiles are, in general, rich in hydrophobic residues, display an increase in flexibility, and have a lower number of secondary stabilizing bonds when compared with homologous proteins from mesophiles. Antifreeze proteins and solutes that decrease the freezing temperature of the cytoplasm are common. The lipids in the membranes tend to be unsaturated to increase fluidity. Growth rates are much slower than those encountered at moderate temperatures. Under appropriate conditions, mesophiles and even thermophiles can survive freezing. Liquid cultures of bacteria are mixed with sterile glycerol solutions and frozen to −80 °C for long-term storage as stocks. Cultures can withstand freeze drying (lyophilization) and then be stored as powders in sealed ampules to be reconstituted with broth when needed.
Macromolecules in thermophiles and hyperthermophiles show some notable structural differences from what is observed in the mesophiles. The ratio of saturated to polyunsaturated lipids increases to limit the fluidity of the cell membranes. Their DNA sequences show a higher proportion of guanine–cytosine nitrogenous bases, which are held together by three hydrogen bonds in contrast to adenine and thymine, which are connected in the double helix by two hydrogen bonds. Additional secondary ionic and covalent bonds, as well as the replacement of key amino acids to stabilize folding, contribute to the resistance of proteins to denaturation. The so-called thermoenzymes purified from thermophiles have important practical applications. For example, amplification of nucleic acids in the polymerase chain reaction (PCR) depends on the thermal stability of Taq polymerase, an enzyme isolated from T. aquaticus. Degradation enzymes from thermophiles are added as ingredients in hot-water detergents, increasing their effectiveness.
Exercise \(\PageIndex{1}\)
- What temperature requirements do most bacterial human pathogens have?
- What DNA adaptation do thermophiles exhibit?
- Some hyperthermophiles can survive autoclaving temperatures. Are they a concern in health care?
Feeding the World... and the World's Algae
Artificial fertilizers have become an important tool in food production around the world. They are responsible for many of the gains of the so-called green revolution of the 20th century, which has allowed the planet to feed many of its more than 7 billion people. Artificial fertilizers provide nitrogen and phosphorus, key limiting nutrients, to crop plants, removing the normal barriers that would otherwise limit the rate of growth. Thus, fertilized crops grow much faster, and farms that use fertilizer produce higher crop yields.
However, careless use and overuse of artificial fertilizers have been demonstrated to have significant negative impacts on aquatic ecosystems, both freshwater and marine. Fertilizers that are applied at inappropriate times or in too-large quantities allow nitrogen and phosphorus compounds to escape use by crop plants and enter drainage systems. Inappropriate use of fertilizers in residential settings can also contribute to nutrient loads, which find their way to lakes and coastal marine ecosystems. As water warms and nutrients are plentiful, microscopic algae bloom, often changing the color of the water because of the high cell density.
Most algal blooms are not directly harmful to humans or wildlife; however, they can cause harm indirectly. As the algal population expands and then dies, it provides a large increase in organic matter to the bacteria that live in deep water. With this large supply of nutrients, the population of nonphotosynthetic microorganisms explodes, consuming available oxygen and creating “dead zones” where animal life has virtually disappeared.
Depletion of oxygen in the water is not the only damaging consequence of some algal blooms. The algae that produce red tides in the Gulf of Mexico, Karenia brevis, secrete potent toxins that can kill fish and other organisms and also accumulate in shellfish. Consumption of contaminated shellfish can cause severe neurological and gastrointestinal symptoms in humans. Shellfish beds must be regularly monitored for the presence of the toxins, and harvests are often shut down when it is present, incurring economic costs to the fishery. Cyanobacteria, which can form blooms in marine and freshwater ecosystems, produce toxins called microcystins, which can cause allergic reactions and liver damage when ingested in drinking water or during swimming. Recurring cyanobacterial algal blooms in Lake Erie (Figure \(\PageIndex{6}\)) have forced municipalities to issue drinking water bans for days at a time because of unacceptable toxin levels.
This is just a small sampling of the negative consequences of algal blooms, red tides, and dead zones. Yet the benefits of crop fertilizer—the main cause of such blooms—are difficult to dispute. There is no easy solution to this dilemma, as a ban on fertilizers is not politically or economically feasible. In lieu of this, we must advocate for responsible use and regulation in agricultural and residential contexts, as well as the restoration of wetlands, which can absorb excess fertilizers before they reach lakes and oceans.

This video discusses algal blooms and dead zones in more depth.
Key Concepts and Summary
- Bacteria are generally neutrophiles. They grow best at neutral pH close to 7.0.
- Acidophiles grow optimally at a pH near 3.0. Alkaliphiles are organisms that grow optimally between a pH of 8 and 10.5. Extreme acidophiles and alkaliphiles grow slowly or not at all near neutral pH.
- Microorganisms grow best at their optimum growth pH. Growth occurs slowly or not at all below the minimum growth pH and above the maximum growth pH.
- Microorganisms thrive at a wide range of temperatures; they have colonized different natural environments and have adapted to extreme temperatures. Both extreme cold and hot temperatures require evolutionary adjustments to macromolecules and biological processes.
- Psychrophiles grow best in the temperature range of 0–15 °C whereas psychrotrophs thrive between 4°C and 25 °C.
- Mesophiles grow best at moderate temperatures in the range of 20 °C to about 45 °C. Pathogens are usually mesophiles.
- Thermophiles and hyperthemophiles are adapted to life at temperatures above 50 °C.
- Adaptations to cold and hot temperatures require changes in the composition of membrane lipids and proteins.
Contributors
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


