20.5: The Phosphorus Cycle
- Page ID
- 69926
Key Points
- Phosphorus, a major component of nucleic acid and phospholipids, also makes up the supportive components of our bones; it is often necessary for growth in aquatic ecosystems.
- Phosphates (PO43−) are sent into rivers, lakes, and the ocean by leaching and natural surface runoff.
- Phosphate-containing ocean sediments slowly move to land by the uplifting of areas of the earth’s surface.
The Phosphorus Cycle
Phosphorus is an essential nutrient for living processes. It is a major component of nucleic acids, both DNA and RNA; of phospholipids, the major component of cell membranes; and, as calcium phosphate, makes up the supportive components of our bones. Phosphorus is often the limiting nutrient (necessary for growth) in aquatic ecosystems.
Phosphorus occurs in nature as the phosphate ion (PO43−). In addition to phosphate runoff as a result of human activity, natural surface runoff occurs when it is leached from phosphate-containing rock by weathering, thus sending phosphates into rivers, lakes, and the ocean. This rock has its origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, in remote regions, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of areas of the earth’s surface (Figure \(\PageIndex{1}\)). Phosphorus is also reciprocally exchanged between phosphate dissolved in the ocean and marine ecosystems. The movement of phosphate from the ocean to the land and through the soil is extremely slow, with the average phosphate ion having an oceanic residence time between 20,000 and 100,000 years.

Figure \(\PageIndex{1}\): In nature, phosphorus exists as the phosphate ion (PO43−). Weathering of rocks and volcanic activity releases phosphate into the soil, water, and air, where it becomes available to terrestrial food webs. Phosphate enters the oceans via surface runoff, groundwater flow, and river flow. Phosphate dissolved in ocean water cycles into marine food webs. Some phosphate from the marine food webs falls to the ocean floor, where it forms sediment.
Marine birds play a unique role in the phosphorous cycle. These birds take up phosphorous from ocean fish. Their droppings on land (guano) contain high levels of phosphorous and are sometimes mined for commercial use. A 2020 study estimated that the ecosystem services (natural processes and products that benefit humans) provided by guano are worth $470 million per year.
Weathering of rocks releases phosphates into the soil and bodies of water. Plants can assimilate phosphates in the soil and incorporate it into organic molecules, making phosphorus available to consumers in terrestrial food webs. Waste and dead organisms are decomposed by fungi and bacteria, releasing phosphates back into the soil. Some phosphate is leached from the soil, entering into rivers, lakes, and the ocean. Primary producers in aquatic food webs, such as algae and photosynthetic bacteria, assimilate phosphate, and organic phosphate is thus available to consumers in aquatic food webs. Similar to terrestrial food webs, phosphorus is reciprocally exchanged between phosphate dissolved in the ocean and organic phosphorus in marine organisms.
The movement of phosphorus from rock to living organisms is normally a very slow process, but some human activities speed up the process. Phosphate-bearing rock is often mined for use in the manufacture of fertilizers and detergents. This commercial production greatly accelerates the phosphorous cycle. In addition, runoff from agricultural land and the release of sewage into water systems can cause a local overload of phosphate. The increased availability of phosphate can cause overgrowth of algae. This reduces the oxygen level, causing eutrophication and the destruction of other aquatic species.
The processes of plant production and decomposition are important for biogeochemical cycling of phosphorus within terrestrial and aquatic ecosystems, just as they are for the nitrogen and carbon cycles. Figure \(\PageIndex{2}\) shows a close up of the phosphorus (and nitrogen) cycling within a wetland controled by plant productivity and decomposition.
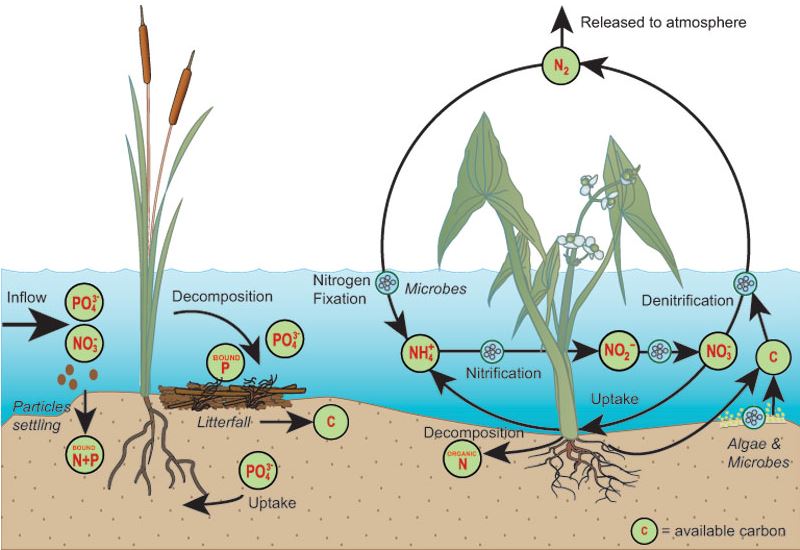
Figure \(\PageIndex{2}\): A Simplified Diagram of Phosphorus and Nitrogen Cycle in a Wetland Source: Kadlec and Knight (1996), CC BY-SA 4.0, via Wikimedia Commons
Contributors and Attributions
Modified by Kyle Whittinghill (University of Pittsburgh) and Melissa Ha from the following sources:
- Biogeochemical Cycles, Energy, and Energy Enters Ecosystems Through Photosynthesis from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Energy Flow through Ecosystems from General Biology by OpenStax (licensed under CC-BY)
- Soil and Sustainability and Biogeochemical Cycles and the Flow of Energy in the Earth System from Sustainability: A Comprehensive Foundation by Tom Theis and Jonathan Tomkin, Editors (licensed under CC-BY). Download for free at CNX.
- Cycling of Matter from AP Environmental Science by University of California College Prep, University of California (licensed under CC-BY). Download for free at CNX.
- Nutrient Cycles from Life Sciences Grade 10 by Siyavula (CC-BY)
Samantha Fowler (Clayton State University), Rebecca Roush (Sandhills Community College), James Wise (Hampton University). Original content by OpenStax (CC BY 4.0; Access for free at https://cnx.org/contents/b3c1e1d2-83...4-e119a8aafbdd).
20.2: Biogeochemical Cycles by OpenStax, is licensed CC BY- General Microbiology Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike

