11.2.2: Mitigating Water Pollution
- Last updated
- Save as PDF
- Page ID
- 72707

- Melissa Ha and Rachel Schleiger
- Yuba College & Butte College via ASCCC Open Educational Resources Initiative
Water pollution can be mitigated through regulation, bioremediation, and watershed management.
Regulation
During the early1900s, rapid industrialization in the U.S. resulted in widespread water pollution due to free discharge of waste into surface waters. The Cuyahoga River in northeast Ohio caught fire numerous times, including a famous fire in 1969 that caught the nation’s attention (figure \(\PageIndex{a}\)). In 1972 Congress passed one of the most important environmental laws in U.S. history, the Federal Water Pollution Control Act, which is more commonly called the Clean Water Act (CWA). The purpose of the Clean Water Act and later amendments is to maintain and restore water quality, or in simpler terms to make our water swimmable and fishable. It became illegal to dump pollution into surface water unless there was formal permission. The CWA regulates pollution from a single source such as that from industry or sewage treatment plants by setting pollution standards (maximum levels of each pollutant that can be in bodies of water or released at a time). United States water quality improved significantly as a result, but as there is still more work to be done.

Remediation
Remediation is the act of cleaning contamination. Biological remediation (bioremediation) is a waste management technique that involves the use of organisms such as plants, bacteria, and fungi to remove or neutralize pollutants from a contaminated site. According to the United States EPA, bioremediation is a “treatment that uses naturally occurring organisms to break down hazardous substances into less toxic or nontoxic substances”. This type of remediation is usually used on organic chemicals but also works on reducing or oxidizing inorganic chemicals like nitrate. Phytoremediation is a type of bioremediation that uses plants to absorb the chemicals over time.
Bioremediation is widely used to treat human sewage and has also been used to remove agricultural chemicals (pesticides and fertilizers) that leach from soil into groundwater. Certain toxic metals, such as selenium and arsenic compounds, can also be removed from water by bioremediation. Mercury is an example of a toxic metal that can be removed from an environment by bioremediation. Several species of bacteria can carry out the biotransformation of toxic mercury into nontoxic forms. These bacteria, such as Pseudomonas aeruginosa, can convert a charged form of mercury (Hg2+) to an uncharged form (Hg), which is less toxic to humans.
Probably one of the most useful and interesting examples of the use of prokaryotes for bioremediation purposes is the cleanup of oil spills. To clean up these spills, bioremediation is promoted by adding inorganic nutrients that help bacteria already present in the environment to grow. Hydrocarbon-degrading bacteria feed on the hydrocarbons in the oil droplet, breaking them into inorganic compounds. Some species, such as Alcanivorax borkumensis, produce surfactants that break the oil into droplets, making it more accessible to the bacteria that degrade the oil. In the case of oil spills in the ocean, ongoing, natural bioremediation tends to occur, inasmuch as there are oil-consuming bacteria in the ocean prior to the spill. Under ideal conditions, it has been reported that up to 80 percent of the nonvolatile components (those that do not readily evaporate) in oil can be degraded within one year of the spill. Researchers have genetically engineered other bacteria to consume petroleum products; indeed, the first patent application for a bioremediation application in the U.S. was for a genetically modified oil-eating bacterium.

There are a number of cost/efficiency advantages to bioremediation, which can be employed in areas that are inaccessible without excavation. For example, hydrocarbon spills (specifically, oil spills) or certain chlorinated solvents may contaminate groundwater, which can be easier to treat using bioremediation than more conventional approaches. This is typically much less expensive than excavation followed by disposal elsewhere, incineration, or other off-site treatment strategies. It also reduces or eliminates the need for “pump and treat”, a practice common at sites where hydrocarbons have contaminated clean groundwater. Using microorganisms for bioremediation of hydrocarbons also has the advantage of breaking down contaminants at the molecular level, as opposed to simply chemically dispersing the contaminant.
Chemical remediation uses the introduction of chemicals to remove the contaminant or make it less harmful. One example is reactive barriers, a permeable wall in the ground or at a discharge point that chemically reacts with contaminants in the water. Reactive barriers made of limestone can increase the pH of acid mine drainage, making the water less acidic and more basic, which removes dissolved contaminants by precipitation into a solid form. Physical remediation consists of removing the contaminated water and either treating it (aka pump and treat) with filtration or disposing of it. All of these options are technically complex, expensive, and difficult, with physical remediation typically being the most costly.
Watershed Management
Watershed management involves reducing chemicals applied to land in the watershed (which will drain into a body of water) and runoff of those chemicals (figure \(\PageIndex{b-c}\)). This strategy is more effective for nonpoint source pollution than setting pollution standards (as the CWA does) because it does not require each source of pollution be identified.

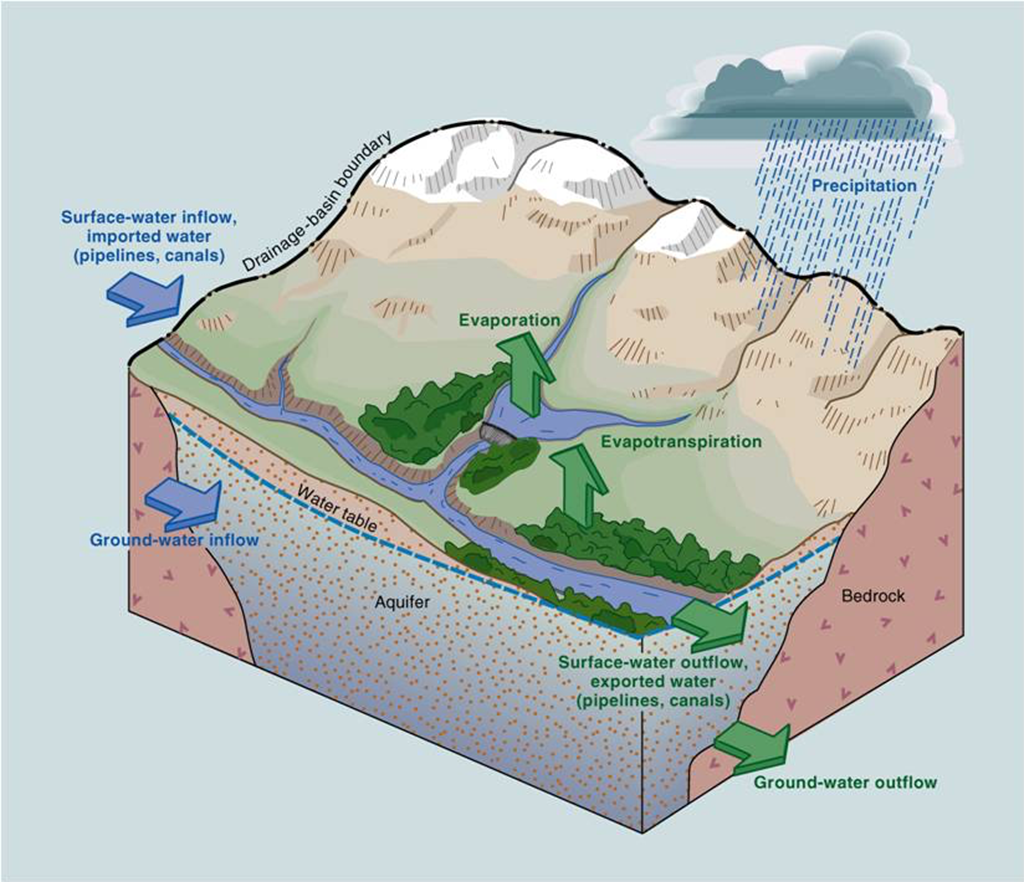
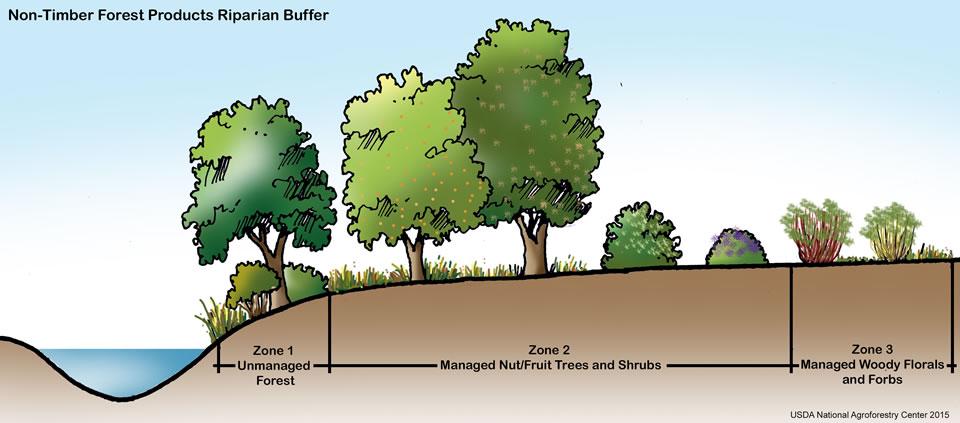
Maintaining or restoring riparian areas (riparian zones) is key to watershed management (figure \(\PageIndex{d}\)). These are areas of land close enough to a body of water to be affected by that body of water, for example, the lush region of vegetation surrounding a river. Riparian areas provide many ecosystem services that promote water quality and limit pollution. The vegetation absorbs nutrients that could otherwise cause eutrophication. It also provide shade, keeping the water cool and increasing its capacity to hold dissolved oxygen. The roots of the vegetation cling to soil, combating erosion. Additionally, roots and low-lying plants slow the flow of run-off, promoting infiltration. High infiltration rates have several benefits: (1) less runoff is available to bring pollution to the river, lake, or bay, (2) pollutants will be filtered from the runoff as it soaks into the ground, and (3) aquifers are replenished.

Watershed management plans typically leave undisturbed riparian vegetation directly adjacent to a body of water (figure \(\PageIndex{e}\)). Next (moving away from the water), could be managed forest followed by agriculture. The most intensive agriculture should be farthest from the body of water, particularly when pesticides and fertilizers are in use.
In urban areas can also be carefully structured to limit water pollution. Rain gardens next to buildings are areas with soil and vegetation that promote infiltration. Permeable pavement, which allows water to pass through it, also promotes infiltration, and reduces runoff, creating fewer opportunities for the water to acquire pollutants from washing over a dirty street.
Attribution
Modified by Melissa Ha from the following sources:
- Water Contamination from An Introduction to Geology by Johnson et al. (licensed under CC-BY-NC-SA)
- Water Treatment and Bioremediation from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)

