13.2: Introduction
- Page ID
- 40233
Like other cells, the osmotic environment surrounding microorganisms can affect them. Osmosis is the net movement of water molecules across a semi-permeable membrane, from an area of low solute (high water activity) concentration to higher solute concentration (lower water activity). Water molecules want to balance the solute concentration on either side of the membrane to a more isotonic state. Osmotic pressure is the physical force to draw water to one side of the membrane or other. In a hypertonic solution (more solutes outside of the cell, higher osmotic pressure) there will be net movement of water out of the cell (in order to create equal osmotic pressure on both sides of the membrane). Plasmolysis, cell shrinkage, may occur. In a hypotonic solution the opposite will happen. There are more solutes inside the cell relative to the solution and the net movement of water will be into the cell, which may result in lysis of the cell.
It’s important to remember that osmosis is relative to the two side of the membrane. When considering a microorganism and its environment, one should consider the inside of the cell relative to the environment. Is the environment hypertonic (higher solutes) or hypotonic (lower solutes) relative to the cell? This will determine the net movement of water.
Net movement of water
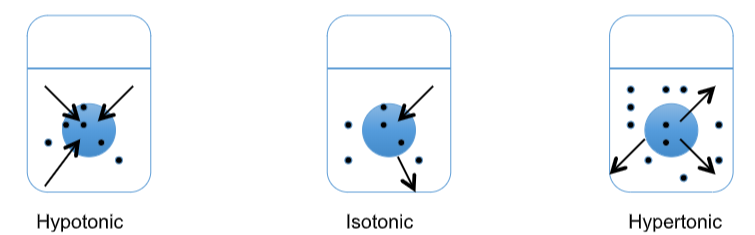
The bacterial cell wall helps those (with a cell wall) maintain their osmotic pressure in a fairly stable environment so that neither plasmolysis nor lysis will occur. Most bacteria live in a narrow range of salinity, usually less than 3% \(\ce{NaCl}\). Halophiles will grow in higher than 3% \(\ce{NaCl}\). Extreme halophiles require a very high salt concentration (up to 30%) and can be found in places like the Dead Sea, Great Salt Lake, etc. These organisms have proteins, enzymes, membranes, etc., that function in the high osmotic environment. In addition, some bacteria have adapted mechanisms to survive in a wider range of osmotic environments and are osmotolerant.
Contributors and Attributions
Kelly C. Burke (College of the Canyons)


