15.3: Carbon Cycle
- Last updated
- Save as PDF
- Page ID
- 94343
Key Points
- Carbon is present in all organic molecules; carbon compounds contain large amounts of energy, which humans use as fuel.
- The biological carbon cycle is the rapid exchange of carbon among living things; autotrophs use carbon dioxide produced by heterotrophs to produce glucose and oxygen, which are then utilized by heterotrophs.
- The biogeochemical cycle occurs at a much slower rate than the biological cycle since carbon is stored in carbon reservoirs for long periods of time.
- Carbon dioxide from the atmosphere dissolves in water, combining with water molecules to form carbonic acid, which then ionizes to carbonate and bicarbonate ions.
- Most of the carbon in the ocean is in the form of bicarbonate ions, which can combine with seawater calcium to form calcium carbonate (CaCO3), a major component of marine organism shells.
- Carbon can enter the soil as a result of the decomposition of living organisms, the weathering of rocks, the eruption of volcanoes, and other geothermal systems.
The Carbon Cycle
Carbon is the fourth most abundant element in living organisms. Carbon is present in all organic molecules, and its role in the structure of macromolecules is of primary importance to living organisms. Carbon compounds contain energy, and many of these compounds from plants and algae have remained stored as fossilized carbon, which humans use as fuel. Since the 1800s, the use of fossil fuels has accelerated. As global demand for Earth’s limited fossil fuel supplies has risen since the beginning of the Industrial Revolution, the amount of carbon dioxide in our atmosphere has increased as the fuels are burned. This increase in carbon dioxide has been associated with climate change and is a major environmental concern worldwide.
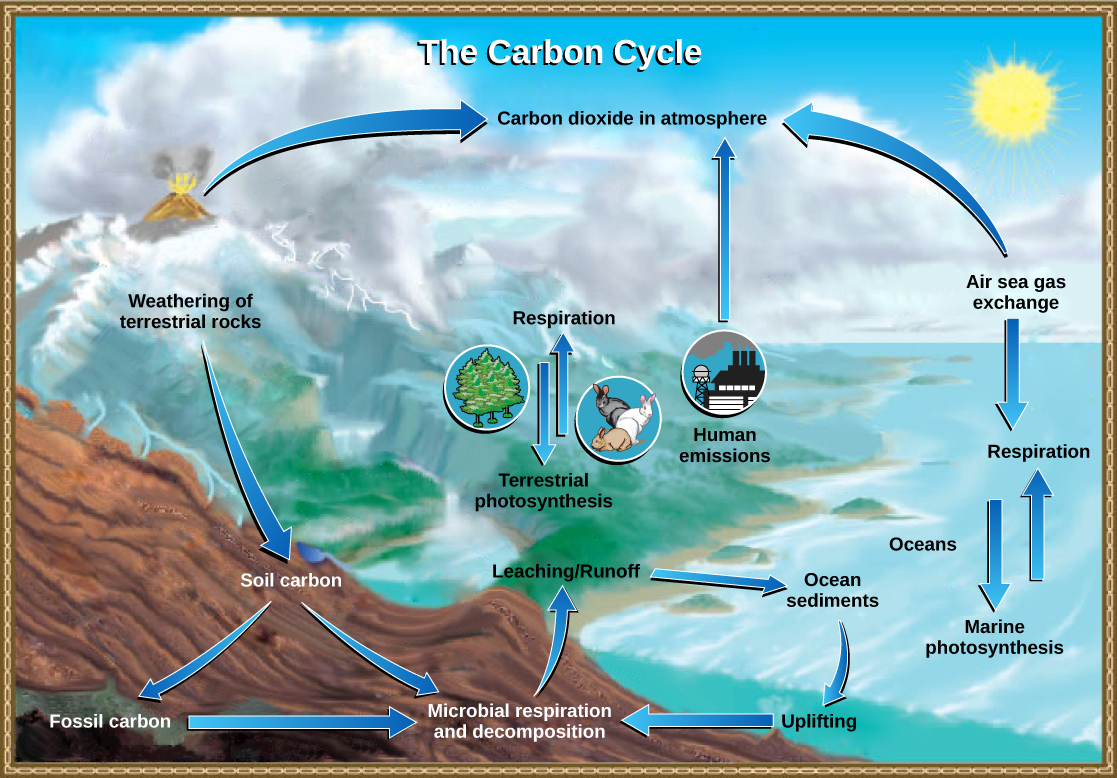
The carbon cycle is most easily studied as two interconnected subcycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes. The entire carbon cycle is shown in Figure \(\PageIndex{1}\). The overall effect is that carbon is constantly recycled in the dynamic processes taking place in the atmosphere, at the surface and in the crust of the earth. The vast majority of carbon resides as inorganic minerals in crustal rocks. Other reservoirs of carbon, places where carbon accumulates, include the oceans and atmosphere. Some of the carbon atoms in your body today may long ago have resided in a dinosaur's body, or perhaps were once buried deep in the Earth's crust as carbonate rock minerals.
Carbon Cycles Quickly between Organisms and the Atmosphere
Cells run on the chemical energy found mainly in carbohydrate molecules, and the majority of these molecules are produced by one process: photosynthesis. Through photosynthesis, certain organisms convert solar energy (sunlight) into chemical energy, which is then used to build other organic molecules like complex carbohydrates (such as starch), proteins and lipids. The energy stored in the bonds to hold these molecules together is released when an organism breaks down food. Cells then use this energy to perform work, such as movement. The energy that is harnessed from photosynthesis enters the ecosystems of our planet continuously and is transferred from one organism to another. Therefore, directly or indirectly, the process of photosynthesis provides most of the energy required by living things on Earth. Photosynthesis also results in the release of oxygen into the atmosphere. In short, to eat and breathe humans depend almost entirely on the organisms that carry out photosynthesis.
Figure \(\PageIndex{2}\): (a) Plants, (b) algae, and (c) certain bacteria, called cyanobacteria, are can carry out photosynthesis. Algae can grow over enormous areas in water, at times completely covering the surface. (credit a: Steve Hillebrand, U.S. Fish and Wildlife Service; credit b: “eutrophication&hypoxia”/Flickr; credit c: NASA; scale-bar data from Matt Russell)
Some organisms can carry out photosynthesis, whereas others cannot. An autotroph is an organism that can produce its own food. The Greek roots of the word autotroph mean “self” (auto) “feeder” (troph). Plants are the best-known autotrophs, but others exist, including certain types of bacteria and algae (Figure \(\PageIndex{2}\)). Oceanic algae contribute enormous quantities of food and oxygen to global food chains. Carbon dioxide is the basic building block that most autotrophs use to build multi-carbon, high-energy compounds, such as glucose. Most terrestrial autotrophs obtain their carbon dioxide directly from the atmosphere, while marine autotrophs acquire it in the dissolved form (bicarbonate, HCO3–).
Heterotrophs are organisms incapable of photosynthesis that must therefore obtain energy and carbon from food by consuming other organisms. The Greek roots of the word heterotroph mean “other” (hetero) “feeder” (troph), meaning that their food comes from other organisms. Even if the organism being consumed is another animal, it traces its stored energy back to autotrophs and the process of photosynthesis. Heterotrophs acquire the high-energy carbon compounds from the autotrophs by consuming them and breaking them down by respiration to obtain cellular energy, such as ATP. The most efficient type of respiration, aerobic respiration, requires oxygen obtained from the atmosphere or dissolved in water. Thus, there is a constant exchange of oxygen and carbon dioxide between the autotrophs (which need the carbon) and the heterotrophs (which need the oxygen). Humans are heterotrophs, as are all animals and fungi. A deer obtains energy by eating plants. A wolf eating a deer obtains energy that originally came from the plants eaten by that deer (Figure \(\PageIndex{3}\)). Using this reasoning, all food eaten by humans can be traced back to autotrophs that carry out photosynthesis.
Figure \(\PageIndex{3}\): The energy stored in carbohydrate molecules from photosynthesis passes through the food chain. The predator that eats these deer is getting energy that originated in the photosynthetic vegetation that the deer consumed. (credit: Steve VanRiper, U.S. Fish and Wildlife Service)
Plants, animals, and other organisms break down organic molecules during the process of aerobic cellular respiration, which consumes oxygen and releases energy, water and carbon dioxide. Carbon dioxide is returned to the atmosphere during gaseous exchange. Another process by which organic material is recycled is the decomposition of dead organisms. During this process, bacteria and fungi break down the complex organic compounds. Decomposers may do respiration, releasing carbon dioxide, or other processes that release methane (CH4).
Photosynthesis and respiration are actually reciprocal to one another with regard to the cycling of carbon: photosynthesis removes carbon dioxide from the atmosphere and respiration returns it (Figure \(\PageIndex{4}\)). A significant disruption of one process can therefore affect the amount of carbon dioxide in the atmosphere.
Figure \(\PageIndex{4}\): This equation means that six molecules of carbon dioxide (CO2) combine with six molecules of water (H2O) in the presence of sunlight. This produces one molecule of glucose (C6H12O6) and six molecules of oxygen (O2).
Cellular respiration is only one process that releases carbon dioxide. Physical processes, such as the eruption of volcanoes and release from hydrothermal vents (openings in the ocean floor) add carbon dioxide to the atmosphere. Additionally, the combustion of wood and fossil fuels releases carbon dioxide. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. The exchange of carbon between the atmosphere and water reservoirs influences how much carbon is found in each.
Carbon Cycles Slowly between Land and the Ocean
The movement of carbon through land, water, and air is complex, and, in many cases, it occurs much more slowly geologically than the movement between living organisms. Carbon is stored for long periods in what are known as carbon reservoirs, which include the atmosphere, bodies of liquid water (mostly oceans), ocean sediment, soil, rocks (including fossil fuels), and Earth’s interior.
As stated, the atmosphere is a major reservoir of carbon in the form of carbon dioxide that is essential to the process of photosynthesis. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. The exchange of carbon between the atmosphere and water reservoirs influences how much carbon is found in each, and each one affects the other reciprocally. Atmospheric carbon dioxide also dissolves in the ocean, reacting with water molecules to form carbonic acid and then dissociating into bicarbonate (HCO3-) and carbonate ions (CO32-). More than 90 percent of the carbon in the ocean is found as bicarbonate ions. Some of these ions combine with calcium ions in the seawater to form calcium carbonate (CaCO3), a major component of the shells of marine organisms. These organisms eventually form sediments on the ocean floor. Over geologic time, the calcium carbonate forms limestone, which comprises the largest carbon reservoir on Earth.

On land, carbon is stored in soil as organic carbon as a result of the decomposition of living organisms or from weathering of terrestrial rock and minerals. This carbon can be leached into the water reservoirs by surface runoff. Partially decomposed plants and algae are sometimes buried and compressed between layers of sediments. After millions of years fossil fuels such as coal, oil, and natural gas are formed. Fossil fuels are considered a non-renewable resource because their use far exceeds their rate of formation. A non-renewable resource is either regenerated very slowly or not at all.
Another way for carbon to enter the atmosphere is from land (including land beneath the surface of the ocean) by the eruption of volcanoes and other geothermal systems. Carbon sediments from the ocean floor are taken deep within Earth by the process of subduction: the movement of one tectonic plate beneath another. The ocean sediments are subducted by the actions of plate tectonics, melted and then returned to the surface during volcanic activity. Carbon is released as carbon dioxide when a volcano erupts or from volcanic hydrothermal vents.Plate tectonics can also cause uplifting, returning ocean sediments to land.
Importance of the Carbon Cycle
The carbon cycle is crucially important to the biosphere. If not for the recycling processes, carbon might long ago have become completely sequestered in crustal rocks and sediments, and life would no longer exist (Figure \(\PageIndex{5}\)). Photosynthesis not only makes energy and carbon available to higher trophic levels, but it also releases gaseous oxygen (O2). Gaseous oxygen is necessary for cellular respiration to occur. Photosynthetic bacteria were likely the first organisms to perform photosynthesis, dating back 2-3 billion years ago. Thanks to their activity, and a diversity of present-day photosynthesizing organisms, Earth’s atmosphere is currently about 21% O2. Also, this O2 is vital for the creation of the ozone layer, which protects life from harmful ultraviolet radiation emitted by the sun. Ozone (O3) is created from the breakdown and reassembly of O2.

Figure \(\PageIndex{5}\): Decomposers will break down the organic compounds in this fallen tree at Cliffs of the Neuse State Park in Wayne County, North Carolina, releasing carbon dioxide into the atmosphere. Decomposition ensures that carbon dioxide will be available in the atmosphere for photosynthetic organisms, which then provide carbon for consumers. Image by Gerry Dincher (CC-BY-SA).
The global carbon cycle contributes substantially to the provisioning ecosystem services upon which humans depend. We harvest approximately 25% of the total plant biomass that is produced each year on the land surface to supply food, fuel wood and fiber from croplands, pastures and forests. In addition, the global carbon cycle plays a key role in regulating ecosystem services because it significantly influences climate via its effects on atmospheric CO2 concentrations.
Human Alteration of the Carbon Cycle
Atmospheric CO2 concentration increased from 280 parts per million (ppm) to 413 ppm between the start of industrial revolution in the late eighteenth century and 2020. This reflected a new flux in the global carbon cycle—anthropogenic CO2 emissions—where humans release CO2 into the atmosphere by burning fossil fuels and changing land use. Fossil fuel burning takes carbon from coal, gas, and oil reserves, where it would be otherwise stored on very long time scales, and introduces it into the active carbon cycle. Land use change releases carbon from soil and plant biomass pools into the atmosphere, particularly through the process of deforestation for wood extraction or conversion of land to agriculture. Carbon dioxide is also added to the atmosphere by the breeding and raising of livestock. In 2018, the additional flux of carbon into the atmosphere from anthropogenic sources was estimated to be 36.6 gigatons of carbon (GtC = 1 billion tons of carbon)—a significant disturbance to the natural carbon cycle that had been in balance for several thousand years previously. High levels of carbon dioxide in the atmosphere cause warming that results in climate change.
Contributors and Attributions
Modified by Kyle Whittinghill (University of Pittsburgh) and Melissa Ha from the following sources:
- Biogeochemical Cycles, Energy, and Energy Enters Ecosystems Through Photosynthesis from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Carbon Cycle from Biology by John W. Kimball (licensed under CC-BY)
- Energy Flow through Ecosystems from General Biology by OpenStax (licensed under CC-BY)
- Soil and Sustainability and Biogeochemical Cycles and the Flow of Energy in the Earth System from Sustainability: A Comprehensive Foundation by Tom Theis and Jonathan Tomkin, Editors (licensed under CC-BY). Download for free at CNX.
- Cycling of Matter from AP Environmental Science by University of California College Prep, University of California (licensed under CC-BY). Download for free at CNX.
- Nutrient Cycles from Life Sciences Grade 10 by Siyavula (CC-BY)
Samantha Fowler (Clayton State University), Rebecca Roush (Sandhills Community College), James Wise (Hampton University). Original content by OpenStax (CC BY 4.0; Access for free at https://cnx.org/contents/b3c1e1d2-83...4-e119a8aafbdd).
20.2: Biogeochemical Cycles by OpenStax, is licensed CC BY- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0.
- General Microbiology Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike


