14.3: Activating Macrophages and NK Cells
- Page ID
- 3329
- Describe how TH1 effector cells are able to interact with and activate macrophages.
- Describe how NK cells are able to recognize and destroy infected cells and cancer cells lacking MHC-I molecules.
After interacting with APCs, some naive T4-lymphocytes differentiate into a subset of effector cells called TH1 cells. TH1 cells function primarily to promote phagocytosis of microbes and the killing of intracellular microbes.
Activation of Macrophages
Effector T4-lymphocytes called TH1 cells coordinate immunity against intracellular bacteria and promote opsonization by macrophages. They produce cytokines such as interferon-gamma (IFN-?) that promote cell-mediated immunity against intracellular pathogens, especially by activating macrophages that have either ingested pathogens or have become infected with intracellular microbes such as Mycobacterium tuberculosis, Mycobacterium leprae, Leishmania donovani, and Pneumocystis jiroveci that are able to grow in the endocytic vesicles of macrophages. Activation of the macrophage by TH1 cells greatly enhances their antimicrobial effectiveness (Figure \(\PageIndex{1}\)).
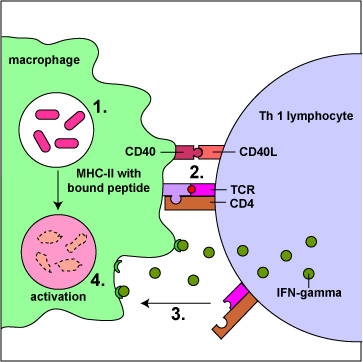
They produce cytokines that promote the production of increases the production of opsonizing and complement activating IgG that enhances phagocytosis (Figure \(\PageIndex{1}\)).
- They produce receptors that bind to and kill chronically infected cells, releasing the bacteria that were growing within the cell so they can be engulfed and killed by macrophages.
- They produce cytokines such as tumor necrosis factor-alpha (TNF-a) that promote diapedesis of macrophages.
- They produce the chemokine CXCL2 to attract macrophages to the infection site.
Activated natural killer T-lymphocytes (NKT cells) also produce large amounts of IFN-gamma to activate macrophages.
Activation of macrophages Increases their production of toxic oxygen radicals, nitric oxide, and hydrolytic lysosomal enzymes enabling the killing of microbes within their phagolysosomes. It also causes the macrophages to secrete cytokines such as TNF-a, IL-1, and IL-12. TNF-a and IL-1 promote inflammation to recruit phagocytic leukocytes. lL-12 enables naive T4-lymphocytes to differentiate into TH1 cells. Moreover activation increases the production of B7 co-stimulator molecules and MHC-1 molecules by macrophages for increased T-lymphocyte activation.
Activation of NK Cells
Cytokines such as interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) produced by TH1 lymphocytes activate NK cells. NK cells are another group of cytolytic lymphocytes, distinct from B-lymphocytes and T-lymphocytes, that participate in both innate immunity and adaptive immunity. NK cells are lymphocytes that lack B-cell receptors and T-cell receptors. They are designed to kill certain mutant cells and virus-infected cells in one of two ways:
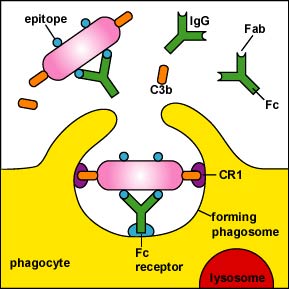
1. NK cells kill cells to which antibody molecules have attached through a process called antibody-dependent cellular cytotoxicity (ADCC) as shown in Figure \(\PageIndex{3}\) , Figure \(\PageIndex{4}\), and Figure \(\PageIndex{5}\) . The Fab portion of the antibody binds to epitopes on the "foreign" cell. The NK cell then binds to the Fc portion of the antibody. The NK cell is then able to contact the cell and by inducing a programmed cell suicide called apoptosis.
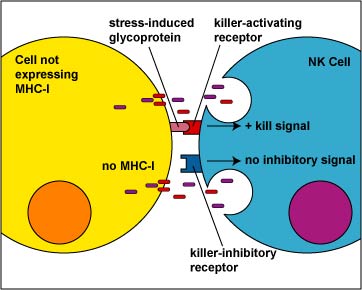
2. NK cells to use a duel receptor system in determining whether to kill or not kill human cells. When cells are either under stress, are turning into tumors, or are infected, various molecules such as MICA and MICB are produced and are put on the surface of that cell. The first receptor, called the killer-activating receptor, can bind to various molecules such as MICA and MICB that are produced and are put on the surface of that cell, and this sends a positive signal that enables the NK cell to kill the cell to which it has bound unless the second receptor cancels that signal. This second receptor, called the killer-ihibitory receptor, recognizes MHC-I molecules that are also usually present on all nucleated human cells. If MHC-I molecules are expressed on the cell, the killer-inhibitory receptor sends a negative signal that overrides the kill signal and prevents the NK cell from killing that cell (Figure \(\PageIndex{6}\)).
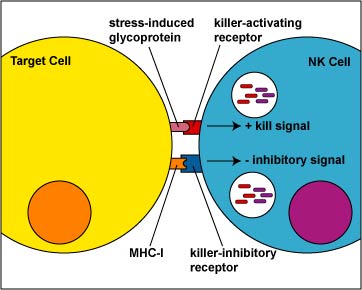
Viruses and malignant transformation can sometimes interfere with the ability of the infected cell or tumor cell to express MHC-I molecules. Without the signal from the killer-inhibitory receptor, the kill signal from the killer-activating signal is not overridden and the NK cell kills the cell to which it has bound (Figure \(\PageIndex{7}\)).
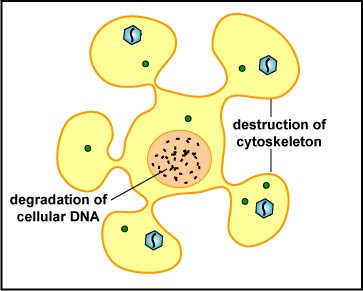
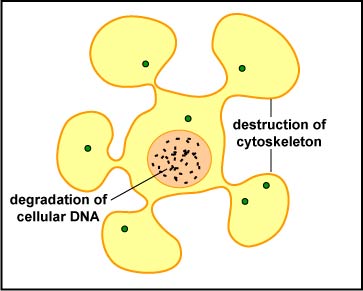
The NK cell releases pore-forming proteins called perforins and proteolytic enzymes called granzymes. Granzymes pass through the pores and activate the enzymes that lead to apoptosis of the infected cell by means of destruction of its structural cytoskeleton proteins and by chromosomal degradation. As a result, the cell breaks into fragments that are subsequently removed by macrophages (Figure \(\PageIndex{5}\)). Perforins can also sometimes result in cell lysis. The distinction between causing apoptosis versus causing cell lysis is important because lysing a virus-infected cell would only release the virions, whereas apoptosis leads to destruction of the virus inside.
NK cells also produce a variety of cytokines, including proinflammatory cytokines, chemokines, colony-stimulating factors, and other cytokines that function as regulators of body defenses. For example, through cytokine production NK cells also suppress and/or activate macrophages, suppress and/or activate the antigen-presenting capabilities of dendritic cells, and suppress and/or activate T-lymphocyte responses.
As with humoral immunity, certain microbes are able to evade to some degree NK cells:
- The cytomegalovirus (CMV) can also trigger its host cell to produce altered MHC-I molecules that are unable to bind viral epitopes, and, therefore, are not recognized by CTLs. However, NK cells are also unable to kill this infected cell because it is still displaying "MHC-I molecules" on its surface.
- CMV also produces microRNAs (miRNAs), small non-coding RNA molecules that down-regulates the production of stress-induced proteins that the killer-activating receptor of NK cells first recognizes. The miRNAs do this by binding to the host cell's mRNA coding for stress-induced proteins ( Figure \(\PageIndex{14}\).3.9). Without this binding there is no kill signal by the NK cell.
- Cytomegalovirus (CMV) and herpes simplex type 1 virus (HSV-1) produce microRNAs (miRNAs), small non-coding RNA molecules that block protein involved in apoptosis, a programmed cell suicide. The miRNAs do this by binding to the host cell's mRNA coding for apoptosis-inducing proteins (Figure \(\PageIndex{9}\)).
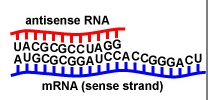
Figure \(\PageIndex{9}\): Antisense RNA (microRNA or miRNA). When an antisense RNA (microRNA or miRNA) that is complementary to a mRNA coding for a particular protein or enzyme binds to the mRNA by complementary base pairing, that mRNA cannot be translated and the protein or enzyme is not made.
Summary
- Effector T4-lymphocytes called TH1 cells coordinate immunity against intracellular bacteria and promote opsonization by macrophages.
- Cytokines produced by TH1 cells promote cell-mediated immunity against intracellular pathogens by activating macrophages and enhancing their antimicrobial effectiveness, increasing the production of opsonizing and complement activating IgG that enhances phagocytosis, and promoting diapedesis and chemotaxis of macrophages to the infection site.
- Activation of natural killer T-lymphocytes (NKT cells) produces large amounts of IFN-gamma to activate macrophages.
- Cytokines such as interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) produced by TH1 lymphocytes activate NK cells.
- Activated NK cells kill cells to which antibody molecules have attached through a process called antibody-dependent cellular cytotoxicity (ADCC).
- Activated NK cells also use a duel receptor system in determining whether to kill or not kill cells such as cancer cells and infected cells that are displaying stress molecules and are not producing MHC-I molecules.
- NK cells kill infected cells and cancer cells by inducing apoptosis, a programmed cell suicide.


