10.5: Other Acellular Infectious Agents: Viroids and Prions
- Page ID
- 3236
- Define viroid and name an infection caused by a viroid.
- Define prion and name 3 protein misfolding diseases that apprear to be initiated by prions.
Viroids and Prions
Viroids are even more simple than viruses. They are small, circular, single-stranded molecules of infectious RNA lacking even a protein coat. They are the cause of a few plant diseases such as potato spindle-tuber disease,cucumber pale fruit, citrus exocortis disease, and cadang-cadang (coconuts).
Prions are infectious protein particles responsible for a group of transmissible and/or inherited neurodegenerative diseases including Creutzfeldt-Jakob disease, kuru, and Gerstmann-Straussler-syndrome in humans, as well as scrapie in sheep and goats, and bovine spongiform encephalopathy (mad cow disease) in cattle and in humans (where it is called new variant Creutzfeldt–Jakob disease humans). The infections are often referred to as transmissible spongiform encephalopathies.
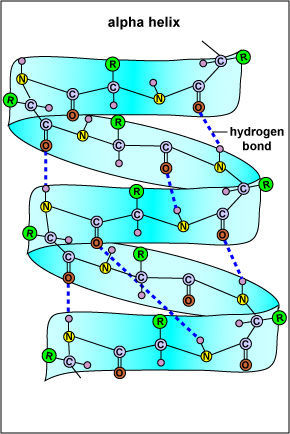
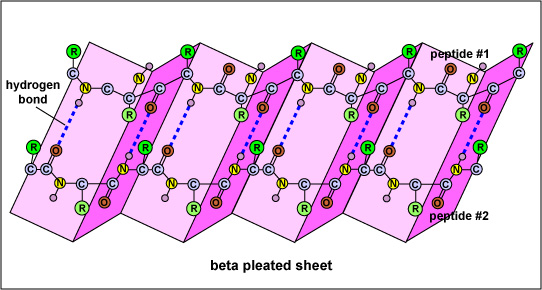
Most evidence indicates that the infectious prion proteins are modified (misfolded) forms of normal proteins coded for by a host gene in the brain. It is thought that the normal prion protein, expressed on stem cells in the bone marrow and on cells that will become neurons, plays a role in the maturation of neurons. In the case of the disease scrapie, the normal prion protein in an animal without the disease has alpha-helices in the proteins secondary structure (Figure \(\PageIndex{1}\)) while the scrapie prion protein in diseased animals has beta-sheets for the secondary structure (Figure \(\PageIndex{2}\)). When the scrapie prion protein contacts the normal protein it causes it to change its configuration to the scrapie beta-sheet form. This suggests that the conversion of a normal prion protein into an infectious prion protein may be catalyzed by the prion protein itself upon entering the brain. Inherited forms may be a result of point mutations that make the prion protein more susceptible to a change in its protein structure.
There is growing evidence that other probable protein misfolding diseases initiated by prions include Alzheimer's disease, Hunington's disease, Parkinson's disease, frontotemporal dementias, amyotrophic lateral sclerosis, and certain cancers.
Summary
- Viroids are small, circular, single-stranded molecules of infectious RNA that cause several plant diseases.
- Prions are infectious protein particles responsible for a group of transmissible and/or inherited neurodegenerative diseases as a result of prion protein misfolding.
- Diseases including Creutzfeldt-Jakob disease Gerstmann-Straussler-syndrome, and mad cow disease.
- There is growing evidence that other probable protein misfolding diseases initiated by prions include Alzheimer's disease, Hunington's disease, Parkinson's disease, amyotrophic lateral sclerosis, and certain cancers.


