41.1: Osmoregulation and Osmotic Balance - Introduction
- Page ID
- 14063
- Describe the process and purpose of osmoregulation
What is osmoregulation?
Doctors typically recommend drinking eight to ten glasses of water a day. This amount is necessary for the proper balance of electrolytes in the human body. The intake is balanced by more or less equal excretion of fluids by urination, defecation, sweating, and, to a lesser extent, respiration. The body’s organs and tissues are immersed in fluid at a constant temperature, pH, and solute concentration, each of which contributes to maintaining the body’s homeostasis. The solutes in body fluids are mainly mineral salts and sugars. Osmotic regulation, or osmoregulation, keeps these solutes at the ideal concentrations. Osmotic homeostasis is maintained despite the influence of external factors such as temperature, diet, and weather conditions.
Osmosis is the diffusion of water across a membrane in response to osmotic pressure caused by an imbalance of molecules on either side of the membrane. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are composed of water plus electrolytes and non-electrolytes. An electrolyte is a solute that dissociates into ions when dissolved in water. A non-electrolyte, in contrast, does not dissociate into ions during water dissolution. Both electrolytes and non-electrolytes contribute to the osmotic balance. The body’s fluids include blood plasma, the cytosol within cells, and interstitial fluid, the fluid that exists in the spaces between cells and tissues of the body. The membranes of the body (such as the pleural, serous, and cell membranes) are semi-permeable: they allow passage of certain types of solutes and water, but not others. Solutions on two sides of a semi-permeable membrane tend to equalize in solute concentration by movement of solutes and/or water across the membrane.
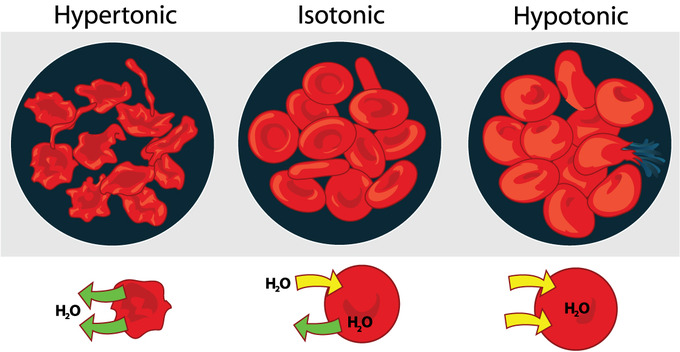
A cell immersed in plain water tends to swell as water diffuses in from the hypotonic or “low salt” solution. In contrast, a cell shrivels when placed in a solution of high salt concentration. The cell loses water, which moves outside to the hypertonic or “high salt” environment. Isotonic cells have an equal concentration of solutes inside and outside the cell; this equalizes the osmotic pressure on either side of the semi-permeable membrane.
Osmoconformers are marine animals which, in contrast to osmoregulators, maintain the osmolarity of their body fluids such that it is always equal to the surrounding seawater. Osmoconformers decrease the net flux of water into or out of their bodies from diffusion. They maintain internal solute concentrations within their bodies at a level equal to the osmolarity of the surrounding medium.
The body is subject to a continual intake and loss of water and electrolytes. Excess electrolytes and wastes that result from osmoregulation are transported to the kidneys and excreted. The process of excretion helps the body maintain osmotic balance.
Need for Osmoregulation
Complex multicellular animals exchange water and nutrients with the environment by consuming food and water, and by excreting sweat, urine, and feces. When disease or injury damage the mechanisms that regulate osmotic pressure, toxic waste or water may accumulate, with potentially dire consequences.
Mammalian systems have evolved to regulate osmotic pressure by managing concentrations of electrolytes found in the three major fluids: blood plasma, extracellular fluid, and intracellular fluid. Water movement due to osmotic pressure across membranes may change the volume of these fluid compartments. Because blood plasma is one of the fluid components, osmotic pressure can directly influence blood pressure and other medical indicators.
Key Points
- Osmoregulation maintains the proper balance of electrolytes in the human body, despite external factors such as temperature, diet, and weather conditions.
- By diffusion of water or solutes, osmotic balance ensures that optimal concentrations of electrolytes and non-electrolytes are maintained in cells, body tissues, and in interstitial fluid.
- Solutes or water move across a semi-permeable membrane, causing solutions on either side of it to equalize in concentration.
- Cells in hypotonic solutions swell as water moves across the membrane into the cell, whereas cells in hypertonic solutions shrivel as water moves out of the cell.
- Water movement due to osmotic pressure across membranes may change the volume of the body’s fluid compartments; therefore, it can directly influence medical indicators, such as blood pressure.
Key Terms
- electrolyte: any of the various ions (such as sodium or chloride) that regulate the electric charge on cells and the flow of water across their membranes
- osmosis: The net movement of solvent molecules from a region of high solvent potential to a region of lower solvent potential through a partially permeable membrane
- osmotic pressure: the hydrostatic pressure exerted by a solution across a semipermeable membrane from a pure solvent


