1.6: Acids and Bases
- Page ID
- 3746
Acids
Acids are substances that donate protons (hydrogen ions, H+) to bases.
Bases
Bases are substances that accept protons from acids.
Example of Acid and Base formation
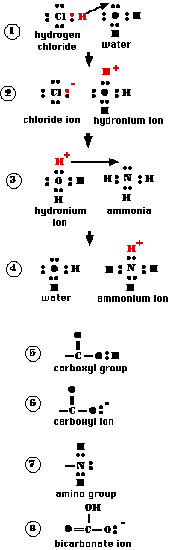 Hydrogen chloride (HCl) is a gas. Its two atoms are held together by a shared pair of electrons. However, the chlorine atom is so much more electronegative than hydrogen, that the bond between them is polar covalent.
Hydrogen chloride (HCl) is a gas. Its two atoms are held together by a shared pair of electrons. However, the chlorine atom is so much more electronegative than hydrogen, that the bond between them is polar covalent.
When hydrogen chloride is bubbled through water, the nucleus of the hydrogen atom leaves and takes up residence at one of the unshared pairs of electrons in the water molecule. However, its electron remains behind still attached to the chlorine atom. "1" This ionization produces:
- a chloride ion (Cl−)
- a hydronium ion (H3O+). "2"
The resulting mixture is called hydrochloric acid.
Now let us bubble ammonia gas (NH3) through the hydrochloric acid. Ammonia molecules have one pair of unshared electrons and these have a greater affinity for a proton than do the unshared electrons in the water molecule. Consequently, the proton shifts again ("3") to form a new ion, the ammonium ion (NH4+) and water ("4").
Because both the HCl molecule and the hydronium ion are proton donors, they meet the definition of an acid.
The water molecule in the first example and the ammonia in the second example accept protons; therefore each is a base.
While HCl is found in living systems (e.g., the gastric juice secreted by the stomach), the most common acids in biology are those containing the carboxyl group ("5").
The proton of the carboxyl group is easily removed forming the carboxyl ion ("6").
Acetic acid (CH3COOH) is a common example of a carboxylic acid. When mixed with water, some of the protons on its -COOH group are attracted to the unshared electron pairs of water molecules. Hydronium ions (H3O+) and acetate ions (CH3COO−) result. Vinegar is a dilute solution of acetic acid.
Ammonia is also found (in low concentrations) in living matter. But the most common bases are those molecules that contain an amino group ("7"). The unshared pair of electrons serves as a proton acceptor, as it does in the ammonia molecule.
Bicarbonate ions ("8") also serve as an important base in living tissue.


