14.4: The Organizer
- Page ID
- 5141
In the embryonic development of a zygote, gradients of mRNAs and proteins, deposited in the egg by the mother as she formed it, give rise to cells of diverse fates despite their identical genomes. But is the embryo fully patterned in the fertilized egg? It is difficult to imagine that the relatively simple gradients in the egg could account for all the complex migration and differentiation of cells during embryonic development. And, in fact, the answer is no. However, once these gradients have sent certain cells along a particular path of gene expression, the stage is set for those cells to begin influencing nearby cells to become increasingly diversified.
In other words, cell-intrinsic signals (established between a nucleus and the particular cytoplasmic environment that cleavage has placed it in) lay the foundation for cell-cell interactions to further guide the cells of the embryo to assume their proper position in the embryo and to differentiate into their final specialized form and function.
Cell-cell interactions could — and probably do — occur in several ways:
- diffusion of a signaling molecule out of one cell and into other cells in the vicinity;
- diffusion of a signaling molecule from one cell into an adjacent cell that then secretes the same molecule to diffuse to the next cell and so on (a "cell-relay" mechanism);
- extension of projections from the plasma membrane of one cell until they make direct contact with nearby cells. This enables proteins embedded in the plasma membrane to serve as signaling molecules.
The Spemann Organizer
In 1924, the Ph.D. student Hilde Mangold working in the laboratory of German embryologist Hans Spemann performed an experiment that demonstrated that the pattern of development of cells is influenced by the activities of other cells and stimulated a search, which continues to this day, for the signals at work. Spemann and Mangold knew that the cells that develop in the region of the gray crescent migrate into the embryo during gastrulation and form the notochord (the future backbone; made of mesoderm). She cut out a piece of tissue from the gray crescent region of one newt gastrula and transplanted it into the ventral side of a second newt gastrula. To make it easier to follow the fate of the transplant, she used the embryo of one variety of newt as the donor and a second variety as the recipient.
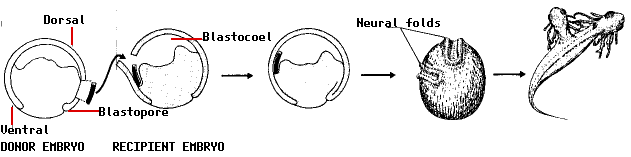
The remarkable results:
- the transplanted tissue developed into a second notochord
- neural folds developed above the extra notochord
- these went on to form a second central nervous system (portions of brain and spinal cord) and eventually
- a two-headed tadpole.
The most remarkable finding of all was that the neural folds were built from recipient cells, not donor cells. In other words, the transplant had altered the fate of the overlying cells (which normally would have ended up forming skin [epidermis] on the side of the animal) so that they produced a second head instead!
Spemann and Mangold used the term induction for the ability of one group of cells to influence the fate of another. And because of the remarkable inductive power of the gray crescent cells, they called this region the organizer. Ever since then, vigorous searches have been made to identify the molecules liberated by the organizer that induce overlying cells to become nerve tissue. One candidate after another has been put forward and then found not to be responsible. Part of the problem has been that not until just recently has it become clear that the organizer does NOT induce the central nervous system but instead it prevents signals originating from the ventral side of the blastula from inducing skin (epidermis) there.
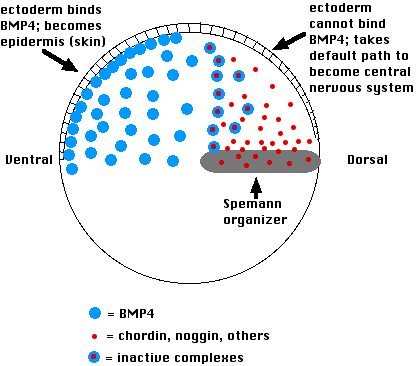
This is how it works:
- Cells on the ventral side of the blastula secrete a variety of proteins such as bone morphogenetic protein-4 (BMP-4)
- These induce the ectoderm above to become epidermis.
- If their action is blocked, the ectodermal cells are allowed to follow their default pathway, which is to become nerve tissue of the brain and spinal cord.
- The Spemann organizer blocks the action of BMP-4 by secreting molecules of the proteins chordin and noggin
- Both of these physically bind to BMP-4 molecules in the extracellular space and thus prevent BMP-4 from binding to receptors on the surface of the overlying ectoderm cells.
- This allows the ectodermal cells to follow their intrinsic path to forming neural folds and, eventually, the brain and spinal cord.
In the Spemann/Mangold experiment, transplanting an organizer to the ventral side provided a second source of chordin. This blocked BMP-4 binding to the overlying ectoderm and thus changed the fate of those cells to forming a second central nervous system rather than skin.
What Organizes the Organizer?
Protein synthesis by the cells of the organizer requires transcription of the relevant genes (e.g., chordin). Expression of organizer genes depends first on Wnt transcription factors. Their messenger RNAs were deposited by the mother in the vegetal pole of the egg. After fertilization and formation of the gray crescent, they migrated into the gray crescent region (destined to become the organizer) where they were translated into Wnt protein.
Its accumulation on the dorsal side of the embryo unleashes the activity of Nodal — a member of the Transforming Growth Factor-beta (TGF-β) family. Nodal induces these dorsal cells to begin expressing the proteins of Spemann's organizer.
A Tail Organizer
One of the distinguishing features of vertebrates is their tail, which extends out behind the anus. French researchers have reported (in the 24 July 2003 issue of Nature) their discovery of a tail "organizer", that is, a cluster of cells in the embryo that induces nearby cells to contribute to the formation of the tail. They worked with the zebrafish, Danio rerio (which also has a head organizer like that of newts). They removed tiny clusters of cells from the ventral part of the blastula (a region roughly opposite where the Spemann-like organizer forms) and transplanted this into a region of the host embryo that would normally form flank. The result: a second tail.
Using a fluorescent label, they were able to show that the extra tail was made not only from descendants of the transplanted cells but also from host cells that would normally have made flank. Three proteins were essential:
- a Wnt protein (establishes the anterior-posterior axis in all bilaterians)
- BMP (establishes the dorsal-ventral axis in all bilaterians)
- Nodal (establishes the left-right axis in all bilaterians)
Patterning the central nervous system in Drosophila
Remarkably, it turns out that proteins similar in structure to the bone morphogenetic proteins and also to chordin are found in Drosophila. The role of BMP-4 is taken by a related protein encoded by the decapentaplegic gene (dpp) and the role of chordin is taken by a related protein called SOG encoded by the gene called short gastrulation.
In fact, these proteins and their mRNAs are completely interchangeable! An injection of the mRNAs for BMP-4 or chordin into the blastoderm of the Drosophila embryo can replace the function of DPP and SOG respectively, and conversely, injections of mRNA for DPP or SOG into the Xenopus embryo mimics the functions of BMP-4 and chordin respectively.
| Xenopus | blocked by chordin | |
|---|---|---|
| and also by noggin | ||
| Drosophila | Decapentaplegic (DPP) | blocked by short gastrulation (SOG) |
| and also by a noggin homolog? |
Dorsal vs Ventral Nerve Cords
Although their actions are similar, the distribution of these proteins in Drosophila differs from that in Xenopus (as well as in mammals and other vertebrates). In Drosophila, DPP is produced in the dorsal region of the embryo and SOG is produced in the ventral region.
However, their actions on overlying cells are the same as in Xenopus; that is, the SOG protein prevents the DPP protein from blocking the formation of the central nervous system. The result in Drosophila is that its central nervous system forms on the ventral side of the embryo, not on the dorsal! And, you may remember that one of the distinguishing traits of all arthropods (insects, crustaceans, arachnids) as well as many other invertebrates, such as the annelid worms, is a ventral nerve cord. Chordates, including all vertebrates, have a dorsal (spinal) nerve cord.
We're halfway done!
Xenopus development (and probably that of animals in general) passes through three rather different (although often overlapping) phases:
- establishing the main axes (dorsal-ventral; anterior-posterior; left-right). This is done by gradients of mRNAs and proteins encoded by the mother's genes and placed in the egg by her.
- establishing the main body parts such as
- the notochord and central nervous system in vertebrates (discussed here and also described in Fro.g Embryology)
- and the segments in Drosophila
- filling in the details; that is, building the various organs of the animal. (Our examples will include the wings, legs, and eyes of Drosophila.)


