4.12: Intermediary Metabolism
- Page ID
- 4650
The immediate source of energy for most cells is glucose. But glucose is not the only fuel on which cells depend. Other carbohydrates, fats and proteins may in certain cells or at certain times be used as a source of ATP. The complexity of the mechanism by which cells use glucose may make you fervently hope that a similarly-constructed system is not needed for each kind of fuel. And indeed it is not.
One of the great advantages of the step-by-step oxidation of glucose into CO2 and H2O is that several of the intermediate compounds formed in the process link glucose metabolism to the metabolism of other food molecules.
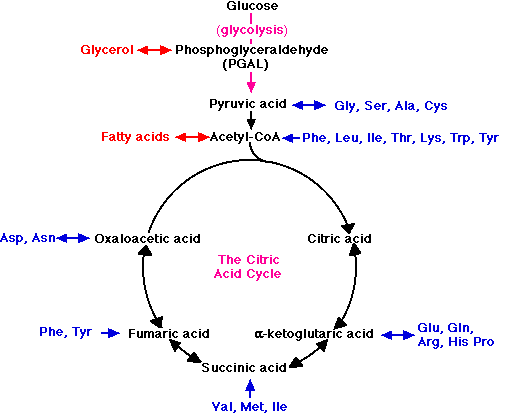
For example, when fats are used as fuel, the glycerol portion of the molecule is converted into PGAL and enters the glycolytic pathway at that point. Fatty acids are converted into molecules of acetyl-CoA and enter the respiratory pathway to be oxidized in the mitochondria. The amino acids liberated by the hydrolysis of proteins can also serve as fuel.
- First, the nitrogen is removed, a process called deamination.
- The remaining fragments then enter the respiratory pathway at several points.
For examples,
- the amino acids Gly, Ser, Ala, and Cys are converted into pyruvic acid and enter the mitochondria to be respired.
- acetyl-CoA and several intermediates in the citric acid cycle serve as entry points for other amino acid fragments (shown in blue).
These links thus permit the respiration of excess fats and proteins in the diet. No special mechanism of cellular respiration is needed by those animals that depend largely on ingested fats (e.g., many birds) or proteins (e.g., carnivores) for their energy supply.
Much of the protein we consume is ultimately converted into glucose (a process called gluconeogenesis) to provide fuel for the brain and other tissues. Although all our foods are interconvertible to some extent, they are not completely so. In other words, no single food can supply all our anabolic needs. We can indeed synthesize many fats from glucose, but certain unsaturated fats cannot be synthesized and must be taken in directly in our diet. These are linoleic acid, linolenic acid, and arachidonic acid. All are unsaturated; that is, have double bonds. Although we can synthesize 11 of the amino acids from carbohydrate precursors, we must obtain 9 others (the "essential amino acids") directly.
Many of the points that connect carbohydrate metabolism to the catabolism of fats and proteins serve as two-way valves (indicated in the figure by double-headed arrows). They provide points of entry not only for the catabolism (cellular respiration) of fatty acids, glycerol, and amino acids, but for their synthesis (anabolism) as well. Thus the catabolic breakdown of starches can lead (through acetyl-CoA and PGAL) to the synthesis of fat.
Fructose presents a special problem
Fructose is produced by the digestion of the disaccharide sucrose (common table sugar) into the monosaccharides glucose and fructose. Both glucose and fructose share the same empirical formula (C6H12O6) and the same caloric content (686 kcal/mole). But the body treats them very differently.
Glucose is taken up and metabolized by all cells to generate ATP by glycolysis and cellular respiration, and excess glucose is preferentially converted into glycogen rather than fats. Fructose is taken up only by liver cells, and excess fructose is converted in fats (fatty acids and glycerol). In the U.S., most soft drinks and prepared foods are now sweetened with high-fructose (60% fructose, 40% glucose) corn syrup. Excessive consumption of these products may well be linked to the growing prevalence in the U.S. of obesity and type 2 diabetes.


