10.3: Butterfly Wing Spots
- Page ID
- 20772
Butterfly wing patterns are organized along Ground Plans, which are compartments for different intervein pattern elements (Figure 1). These pattern elements include eyespots, ellipses, and midlines. The Ground plan, at least for nymphalids, has three symmetry bands: the basal system closest to the body, the central system, the border system, and the margin, which is farthest from the body. Eyespots occur the border band. Eyespots serve a role in predator avoidance and sexual signaling. They come in different sizes, shapes, numbers, and colors (Figure 5). They can also differ within a species based off geography, season, and sex. There are three stages of eyespot development: establishment of eyespot foci in late larval wings, establishment of color rings in early pupae, and pigment synthesis in late pupae.
| Figure 1: Blue Morpho Groundplan by Kinsei Imada. Veins are in blue and pigment spots are in white. Colored regions indicate clonal boundaries. Cells within a clonal boundary are thought to come from the same "mother" cell. Clonal boundaries were estimated by comparing gynandromorphic butterflies to wild type butterflies. Gynandromorphic butterflies have some male cells and some female cells. Cells from a male "mother cell" will all be male and cells from a female "mother cell" will all be female. Since male and female cells have different pigment patterns, gynandromorphic butterflies allow us to figure out clonal boundaries. |
Stages of eyespot development
Establishment of eyespot foci in late larval wings is the first stage of eyespot development. The location of eyespot foci are determined by the genes Antennapedia (Antp), Notch (N), and Distalless (Dll) in Bicyclus anynana. These interact with the transcription factor Spalt. Distalless has different functions in different butterflies. In B. anynana, it promotes eyespot development but in Junonia coenia and Vanessa cardui, two other nymphalid butterflies, it represses it, suggesting that evolution may act even at early stages of eyespot development. Establishment of eyespot foci occurs in four steps: margin and intervein expression, midline patterning, focal determination, and focal maturation.
Notch functions in defining dorsal-ventral boundaries, it defines intervein tissue through lateral inhibition with its ligand Delta. Notch upregulation followed by the activation of Distalless is an early event for the development of eyespots. Notch is upregulated in discrete focal pattern and Distalless is upregulated in intervein midlines (Figure 2). Notch expression tends to increase over time because of a positive feedback mechanism. The focal Notch upregulation precedes Distalless activation with lag time of 1.5 stages which is about 12-24 hours.
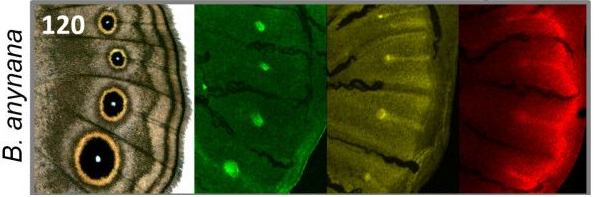 |
Figure 2: Gene expression pre-patterns eyespots. Antennapedia (green) and Notch (yellow) are expressed in foci corresponding to eyespots. Dll (red) and Notch are expressed in intervein midlines. Dll is additionally expressed in the wing margin. Figure from Saenko et al, 2011 under a CC BY 2.0 license. |
Figure 2 shows the spatial and temporal patterns of Antp, Notch and Distalless in late last instar wing marginal discs of three different butterflies. It shows that there is a perfect correlation between presence of eyespots and late last instar Notch and Distalless focal expression. In genetic experiments, researchers reduced levels of Notch or Distalless, which reduced or eliminates two specific eyespots in the hindwing.
Establishment of color rings is the second step of eyespot development. There is currently no confirmed model, but the most popular theory is that there is a morphogenic gradient coming from the eyespot foci. Epidermal scale cells at different distances respond to signal using transcription factors called Engrailed and Spalt. This stage occurs during pupal development. Though not confirmed how this happens, research shows that the expression of both Distalless and Spalt corresponds with adult black scales, and the expression of both Distalless and Engrailed corresponds with gold scales.
Pigment synthesis is the third step of butterfly eyespot development. Figure 4 shows the biochemical pathway of pigment synthesis in B. anynana. As mentioned before, Spalt and Distalless corresponded to black scales, and Engrailed and Distalless corresponded to gold scales. Melanin biosynthesis pathways allow for the pigmentation of these eyespots.
|
Figure 4: Melanin biosynthesis pathways for pigment synthesis in B. anynana. Figure from Matsuoka et al, 2018 and published under a CC BY 4.0 license. |
There are five products of melanin biosynthetic pathways, dopa-melanin, dopamine-melanin, pheomelanin, NBAD, and NADA. Dopa-melanin and dopamine-melanin (the eumelanins) are black and brown pigments, respectively. Pheomelanin is a reddish-yellow pigment, NBAD sclerotin has a yellow color, and NADA sclerotin is colorless. Black scales have a combination of dopa-melanin and dopamine-melanin, which are black and brown pigments. Gold scales have a combination of pheomelanin, dopamine-melanin, and NBAD sclerotin, which are reddish-yellow, brown, and yellow pigments.
Plasticity in eyespot development
Bicyclus anynana eyespots vary in size based on season. Environmental temperature during larval and pupal stages of development leads to changes in ecdysone hormone levels, which regulates eyespot size. This plasticity has likely evolved due to seasonal predation. During the wet season, B. anynana have higher levels of ecdysone and larger eyespots, which help them avoid predation. During the dry season, ecdysone levels drop as do eyespot sizes. Hindwing eyespots display higher levels of plasticity in overall size, center size, and center brightness than forewing eyespots, since the ecdysone receptors in hindwing eyespots are much more sensitive than those in forewing eyespots. The result of this is that slight changes in ecdysone levels affect hindwing eyespot development in a much higher degree than forewing eyespot development.
The evolution of eyespots
Eyespots are present in Nymphalidae. They have convergently evolved in butterflies and moths. In butterflies, they first originated along the dorsal midline, as dorsal fin, then appeared as a pair of anterior pectoral fins, and finally in a more posterior region of the body as pelvic fins. Eyespots replaced the simpler pattern elements that already existed at those locations. The simple colored spot became a multicolored and multi-ringed eyespot.
|
Figure 5: A hypothesis for the evolution of eyespots. Novel features (gene expression, gene networks, and morphological features) are marked where they evolved along the lepidopteran lineage. This hypothesis posits the co-option of discal-cell eyespots (as seen in saturniid butterflies) into border spots and border eyespots following the invention of Spalt regulation of melanin production in scales. Figure from Monteiro et al, 2006 originally published under a CC BY-2.0 license. |
The three selective pressures on eyespots are camouflage, anti-predatory defense, and sexual display. Eyespots are a form of camouflage because they look like vertebrate eyes. The selective pressure of anti-predatory defense and sexual display are often at odds with each other, Bicyclus species overcome this with signal partitioning. Signal partitioning is when visual signals are separated to different parts of the body, and signal displays are restricted to appropriate spatio-temporal conditions. Bicyclus species do this by folding their wings, which hides the dorsal wing surface and exposes the ventral wing surface. To deal with the selective pressure of anti-predatory defense, eyespots are generally on the border of the wing. They are far from the body, so the predators attack the wing instead of the body, allowing the butterfly to escape. Anti-predatory defense places huge selective pressure on large ventral eyespots on the forewing and hindwing because to scare off potential predators, Bicyclus species fold their wings. By exposing the ventral wing surface, there is selection for larger ventral eyespots. On the other hand, during sexual display, males open up their wings to expose the dorsal wing surface. Females prefer intact dorsal forewing eyespots in B. anynana, since it indicates that the eyespots have UV reflective pupils. Sexual display thus select for more intact dorsal forewing eyespots. Figure 6 shows the differences in ventral and dorsal wing surfaces in four different Bicyclus species. Ventral wing surfaces are shown on the left, and dorsal wing surfaces are shown on the right. Ventral eyespots are much bigger than the dorsal eyespots, which shows that signal partitioning does occur with anti-predatory defense selecting for larger ventral eyespots and sexual display selecting for more intact dorsal forewing eyespots. Dorsal and forewing characters are also more likely to change through evolutionary time compared to ventral and hindwing characters because mate selection is a stronger selection pressure than anti-predatory defense.
|
Figure 6: Ventral and dorsal eyespots in four different Bicyclus species. Ventral forewings and hindwings are shown on the left, and dorsal forewings and hindwings are shown on the right. Figure from Oliver et al, 2009. Used with permission. |
References
- Mateus, A., Marques-Pita, M., Oostra, V., Lafuente, E., Brakefield, P. M., Zwaan, B. J., & Beldade, P. (2014). Adaptive developmental plasticity: Compartmentalized responses to environmental cues and to corresponding internal signals provide phenotypic flexibility. BMC Biology,12, 97. doi:10.1186/s12915-014-0097-x
- Matsuoka, Y., & Monteiro, A. (2018). Melanin pathway genes regulate color and morphology of butterfly wing scales. Cell Reports,24(1), 56-65. doi:10.1016/j.celrep.2018.05.092
- Monteiro, A., Chen, B., Oliver, J. C., Ramos, D. M., Tong, X., Guo, M., . . . Kamal, F. (2013). Distal‐Less Regulates Eyespot Patterns and Melanization in Bicyclus Butterflies. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution,320(5), 321-331. doi:10.1002/jez.b.22503
- Monteiro, A., Tong, X., Bear, A., Liew, S. F., Bhardwaj, S., Wasik, B. R., . . . Prudic, K. L. (2015). Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs. PLOS Genetics,11(9). doi:10.1371/journal.pgen.1005529
- Monteiro, A. (2015). Origin, Development, and Evolution of Butterfly Eyespots. Annual Review of Entomology,60, 253-271. doi:10.1146/annurev-ento-010814-020942
- Oliver, J. C., Robertson, K. A., & Monteiro, A. (2009). Accommodating natural and sexual selection in butterfly wing pattern evolution. Proc Biol Sci,276, 2369-2375. doi:10.1098/rspb.2009.0182
- Reed, R. D., & Serfas, M. S. (2004). Butterfly Wing Pattern Evolution Is Associated with Changes in a Notch/Distal-less Temporal Pattern Formation Process. Current Biology,14, 1159-1166. doi:10.1016/j.cub.2004.06.046
- Saenko, S. V., Marialva, M. S., & Beldade, P. (2011). Involvement of the conserved Hox gene Antennapedia in the development and evolution of a novel trait. EvoDevo,2, 9. doi:10.1186/2041-9139-2-9
- Sekimura, T., & Nijhout, H. F. (2017). Diversity and evolution of butterfly wing patterns: An integrative approach. Singapore: Springer Open. doi:10.1007/978-981-10-4956-9
- Monteiro, A., Glaser, G., Stockslager, S., Glansdorm, N., Ramon, D. (2006) Comparative insights into questions of lepidopteran wing pattern homology BMC Developmental Biology 6:52 https://doi.org/10.1186/1471-213X-6-52


