7.1: Pollution in Its Many Forms
- Page ID
- 26792
Rachel Carson’s 1962 book, Silent Spring, described the dangers of pollution—pesticide pollution in particular—with a clarity that captured the public’s attention for many years afterwards. Carson, an American biologist, was particularly successful in drawing attention to biomagnification (also called bioaccumulation), a process through which pesticides and other toxins accumulate and become more concentrated in animals at higher levels of the food chain (Figure 7.1). Her work drew on research that found that dichlorodiphenyltrichloroethane (DDT), sprayed on crops to kill pest insects and on water bodies to kill malaria mosquito (Anopheles spp.) larvae, was also harming non-target organisms that consumed insects and fish exposed to DDT. Of note is that non-target organisms high on food chains, particularly fish-eating birds, such as eagles, pelicans, and egrets, often had high levels of DDT concentrated in their tissues. The affected birds were generally weakened, and the shells of their eggs were thin and prone to cracking during incubation. Consequently, bird populations declined dramatically in areas where DDT was used, as adults died and failed to raise young.

In the 1970s, many industrialized countries recognized the dire situation and banned the use of DDT, which eventually allowed for the partial recovery of the affected bird populations. Unfortunately, while some countries have switched to safer alternatives (e.g. Hargrove, 2003), DDT continues to be widely used in Africa to control malaria mosquito, tsetse fly (Glossina spp.), and other disease vectors. Researchers recently observed complete absences of breeding fish-eating birds in some African wetlands, and some of the highest-ever recorded DDT levels in seed-eating birds (Bouwman et al., 2013). This is cause for concern, not only for wildlife, but also for the long-term effects on people, particularly the consumers of the food products exposed to these chemicals (e.g. Manaca et al., 2011) and the workers who handle these chemicals in the field.
Pollution does not always lead to immediate mortality, but instead can have sublethal impacts that compromise organisms’ fitness over time, with population declines as the end result.
DDT is however not the only form of pollution we battle today. With the impacts of a growing human population becoming gradually more pervasive, pollution is compromising water, soil, and air quality at rates faster than ever before. Some forms of pollution can be highly visible, and with dramatic consequences (Figure 7.2). But importantly, there are many less detectable forms of pollution. While it may not always lead to immediate mortality, these insidious forms of pollution have sublethal impacts that compromise organisms’ fitness over time, with early death and population declines still being the end result. Responding to the silent threats of subtle and easily-overlooked pollution is often delayed, especially when the negative effects are felt only years after exposure. In their totality, pesticides and other pollutants claim 1.4–2.2 million human lives in Africa each year; globally, they claim 9 million lives, which is over three times more than the total impact of AIDS, malaria, and tuberculosis, together (Landrigan et al., 2018). Yet we continue to tolerate these threats, in part because the impact of pollution on our health is not always that apparent, especially when pollution deaths are expressed as a stroke, heart disease, respiratory infections, diarrhoea, or cancer, among other health issues.

One of the most challenging aspects when trying to prevent pollution is identifying the source. Many forms of pollution can easily be transported away from their source through the air, via rivers, even in groundwater. This transport of pollutants (called pesticide drift in the case of pesticides) means that a substantial burden (perhaps as much as 95%, Miller, 2004) of impacts are being felt by non-target species, including economically important non-target organisms. For example, pesticide drift from cotton fields in Benin has caused extirpations of freshwater fish (Agbohessi et al., 2015), while beneficial pollinating insects are also often negatively impacted (Pettis et al., 2013). Studies on fish in Nigeria (Adeogun et al., 2016), large mammals in South Africa (Bornman et al., 2010), and frogs in Kenya (Hayes and Menendez, 1999) have shown that beneficial organisms that survive this secondary pesticide exposure have disrupted reproductive and endocrine systems, and hence reduced fitness. Even humans may be exposed to secondary poisoning from pesticides, as toxic pesticide levels have been found in edible oysters and mussels in Ghana (Dodoo et al., 2013), prawn in Côte d’Ivoire (Roche and Tidou, 2009), and even chickens in South Africa (Barnhoorn et al., 2009).
To make matters worse, many pollutants take many years to biodegrade (i.e. break down in nature), and thus continue to pose a threat to wildlife and humans long after entering the environment. One important class of such long-lived pollutants is persistent organic pollutants (POP). Several types of pesticides qualify as POPs, which are prone to bioaccumulation and drift. The most famous POP is DDT; in the USA, biologist continue to see eggshell thinning and bird deaths, nearly 50 years after DDT was banned in that country (Burnett et al., 2013). This is a concern in places like Ethiopia’s Lake Koka, where recent studies have found DDT residues in every sample of fish tissue (from several different species) tested (Deribe et al., 2011). More information on POPs, many which are banned from use by signatories of the Stockholm Convention on Persistent Organic Pollutants, can be found on the Stockholm Convention website (http://pops.int).
There are also many types of persistent inorganic pollutants that find their way into the environment on a daily basis. One important class of persistent inorganic pollutants that also bioaccumulate is heavy metals; these include mercury, cobalt, copper, lead, and arsenic. A study from Zambia traced cobalt contamination in living trees to soil pollution from mining activities that occurred the mid-1970s (Mihaljevič et al., 2011). Some everyday products can also persist in the environment. For example, an aluminium can takes about 200 years to break down, while a plastic bag takes between 100–1,000 years to break down. The continued use of these products should thus raise alarm to anyone concerned about the environment and human health. But it also provides opportunities for any person to contribute to conservation by reducing use of these products and reusing/recycling those products that find their way into the supply chain.
Many pollutants take many years to biodegrade, and thus continue to pose a threat to wildlife and humans long after entering the environment.
Water pollution
Water pollution, the accidental or intentional dumping of pesticides; herbicides; oil products; fertilisers; sewage; industrial waste; detergents; and other foreign chemicals and objects into aquatic environments, is arguably the biggest current pollution concern in Africa (Prüss-Ustün et al., 2016; Landrigan et al., 2018).
The dumping of products containing heavy metals into aquatic environments is particularly concerning because heavy metals are toxic even in small concentrations, and likely to biomagnify. When aquatic organisms process contaminated water, they absorb or ingest the heavy metals along with other essential nutrients. With each additional step along the food chain, organisms ingest and accumulate increasingly higher concentrations of these toxic elements (see Figure 7.1). In this way, even small amounts of heavy metals can become lethal across several levels of the food web over time. Biomagnification is especially a concern with long-lived predatory marine fishes that people consumed as food, such as swordfish (Xiphias gladius, LC), marlins, sharks, and some tunas and sea basses. For example, mercury (emitted mainly during fossil fuel use), lead, and arsenic have bioaccumulated so much in sharks off South Africa that many species are now considered unsafe for human consumption (McKinney et al., 2016; Bosch et al., 2016; Merly et al., 2019). Recent studies also found unsafe levels of mercury in freshwater fish from regions as wide as Central Africa’s Great Lakes (Campbell et al., 2008), Ethiopia’s Lake Awassa (Desta et al., 2006), and several reservoirs in West Africa (Quédraogo and Amyot, 2013).
Because of biomagnification, many long-lived predatory marine fishes are now considered unsafe for human consumption.
Oil pollution involves the release of petroleum products into the environment, which can originate from damaged ships, failed drilling rigs, leaking offshore platforms, or other unexpected events. The released oil causes mammals and birds to lose the insulating abilities of their fur and feathers, leaving those animals vulnerable to hypothermia and drowning. Other aquatic animals, including fish and shellfish, may ingest oil products, causing them to sicken and die. Because of the way oil is extracted and transported, marine ecosystems are particularly at risk. Furthermore, because of the massive amount of oil that are involved in oil extraction and transport, an oil pollution event often represents a serious ecological disaster (Figure 7.3). Africa has been hit hard by oil spills in recent years, particularly around oil-producing countries like Angola and Nigeria, and along shipping lanes passing along the coasts of Namibia, South Africa, and Mozambique. Nigeria is perhaps the biggest victim of oil spills; between 1976 and 2001, there were an estimated 6,817 oil spills around Africa’s largest wetland, the Niger Delta (UNDP, 2006)! These oil spills have destroyed thousands of hectares of mangrove swamps, estuarine wetlands, and other coastal ecosystems, causing severe hardship to marginalised local communities who depended on those areas for subsistence fishing and farming (Fentiman and Zabbey, 2015).


Plastic pollution is fast becoming a ubiquitous threat to Africa’s environment, its wildlife, and its people. To visualise the magnitude of the problem, consider that there are more than 1.6 trillion pieces of plastic, collectively weighing over 70,000 tonnes, currently floating in the Atlantic and Indian Oceans surrounding Africa (Eriksen et al., 2014). While many of these plastic items were dumped directly in the ocean, many also have a terrestrial origin. For example, if someone throws a plastic wrapper on a sidewalk, there is a good chance that the wrapper will find its way into a nearby stream at some point, carried by wind or rain runoff. From here, the wrapper will float along various streams and rivers until it reaches the ocean. A recent review found that 88–95% of plastics floating into the world’s oceans originated from just 10 rivers, which include West Africa’s Niger River and East Africa’s Nile River (Schmidt et al., 2017; Lebreton et al., 2017). In the process, thousands of seabirds, dolphins, whales, turtles, seals and fish die each year from suffocation or starvation after ingesting plastics and other pieces of trash that they confused with food (Wilcox et al., 2015). This plastic pollution also impacts humans: researchers recently found microfibers (many of which are plastic) in over 80% of tap water samples from Uganda (Kosuth et al., 2017), as well as food-grade commercial sea salt originating from South Africa (Karami et al., 2017).
There are more than 1.6 trillion pieces of plastic, collectively weighing over 70,000 tonnes, currently floating in the Atlantic and Indian Oceans surrounding Africa.
Some of the biggest impacts from plastic pollution are caused not by visible scraps of plastic, but by microplastics, the collective name for plastic particles smaller than 1 mm (some are microscopic). Microplastics may originate from the breakdown of larger pieces of plastic and polystyrene products, or they may be manufactured intentionally small, such as beads added to cosmetics and other personal care products that are flushed down drains after use. Because microplastics are so small, they easily pass through the standard filters used at sewage treatment plants. Consequently, microplastics generally end up in the aquatic environment, where they are unintentionally consumed by crustaceans (crabs, lobsters, and krill), molluscs (mussels, oysters, and clams), echinoderms (sea stars, sea urchins, sea cucumbers), and baby fish. This consumption can block or damage the victim’s digestive and respiratory systems, cause reduced food uptake by creating a false sense of satiation, or even poison animals through leeching of synthetic chemicals. Each of these threats increases death rates and lowers reproductive rates (Sussarellu et al., 2016). Just as with the biomagnification we discussed earlier, the consumption of microplastics also affects other consumers (including humans), because the small organisms that ingest the microplastics are often food for other animals, allowing plastic pollution to move through an entire food chain. For example, a recent study from Lake Victoria found microplastics imbedded in the digestive tracts of perch and tilapia bought at a local market and meant for human consumption (Biginagwa et al., 2016). Because microplastics are so hard to remove once in an ecosystem, the best method for their containment may be to reduce plastic use, to ban products containing microplastics, or to develop microplastics that are biodegradable within a reasonable timeframe. But for this to happen, there is a need to educate the public and lawmakers (Galloway and Lewis, 2016) about the dangers posed by this threat to the environment and local economies.
Nutrient pollution, caused in part by excessive fertiliser use, can led to eutrophication, famous for causing algae blooms, aquatic dead zones, and fish kills.
Nutrient pollution represents another growing threat to Africa’s aquatic environments. Many lakes, streams, and other freshwater and marine environments naturally contain low concentrations of essential nutrients, such as nitrates and phosphates. In order to survive, the species living in these nutrient-poor waters must then be adapted to this natural nutrient scarcity. However, raw sewage, agricultural fertilisers, concentrated animal feeding operations, and industrial processes release large amounts of additional nitrates and phosphates into the environment, which are washed into the aquatic environment. Minor additions of essential nutrients stimulate plant growth, providing more food for organisms at higher trophic levels. However, at high concentrations, the system become subjected to nutrient pollution.
One of the worst outcomes of nutrient pollution is eutrophication. During eutrophication, surface algae grow so rapidly (known as an algae bloom) that it starts blocking sunlight from reaching aquatic organisms below the surface. Because each individual alga is short-lived, their rapid growth also adds large amounts of decaying matter to the environment. In response, decomposers that feed on the dead algae can become so abundant that they consume most of the water’s dissolved oxygen. Without oxygen and sunlight, aquatic plant and animal life may die off in large numbers. The resultant dead zones are sometimes visibly in the form of fish kills, with large numbers of dead fish floating on the surface of the affected water body. The organisms that die during this process is generally also toxic to humans because of bacteria build-up and other imbalances. Eutrophication is an increasingly common problem in Africa; for example, a recent review found that 41–76% of South Africa’s lakes may be eutrophic (Harding, 2015). Eutrophication has already negatively impacted Africa’s tourism and fisheries sectors (Nyenje et al., 2010), and even led a temporary shutdown of water supplies on the Kenyan side of Lake Victoria (Sitoki et al., 2012). Preventing further eutrophication should thus be a high priority—not only will it prevent harmful algae blooms but may even play an important role in controlling invasive aquatic plants such as the water hyacinth (Eichhornia crassipes) (Coetzee and Hill, 2012; Bownes et al., 2013).
Groundwater pollution—the release of pollutants into aquifers and other sources of groundwater—is also becoming a serious issue across Africa. This type of pollution generally originates from landfills, on-site sanitation systems, leaking sewage systems, mining leachate, agriculture runoff (fertiliser, pesticides, animal waste, etc.), and other types of waste dumping. The pollutants may sometimes be released directly into aquifers; however, more often the contaminants and pathogens leak into the soil, from where it seeps into groundwater.
Because fracking poses many serious risks, governments across the world have banned the practice from their lands.
One of the most important emerging threats to groundwater in Africa is hydrological fracturing or fracking, in short. During this process, pressurised liquids that contain suspended particles and thickening agents are blasted into rock formations deep underground to break them open. When the pressure and liquids are removed, the suspended particles keep the fractures open, which enables extraction of natural gas and petroleum. While fracking was initially hailed as a method to access previously inaccessible fossil fuels, scientists subsequently found that it poses a wide variety of very serious environmental and health risks. Most importantly, the liquids used in fracking contain toxic chemicals which pose a high risk for groundwater pollution (Osborne et al., 2011), which in turn lead to miscarriages and birth defects (McKenzie et al., 2014), cancer (McKenzie et al., 2012), as well as skin and respiratory diseases (Rabinowitz et al. 2015). In addition, fracking increases greenhouse gas emissions (Howarth, 2014) and induces infrastructure-damaging earthquakes (Ellsworth, 2013). Because of these myriad serious risks, several national governments in Europe, and several local governments in the USA, UK, Canada, and Australia have banned the practice from their lands (https://keeptapwatersafe.org/global-bans-on-fracking). In contrast, and despite opposition from civil society, several countries in Africa (e.g. South Africa: Roelf, 2016; Botswana: Barbee, 2015) recently approved this harmful practice.
Air pollution
In the past, people and industries thought that the atmosphere was so vast that any gases or particles released into the air would disperse and dilute to the point that they would post no ill effects. But as air quality has diminished over time, scientists have documented that air pollution can cause irreparable harm to ecosystems and human health, often far from the original sources. A striking example comes from West Africa’s Lake Chad, which shrank by 95% between 1963 and 1998 (Figure 7.4). Experts generally thought that the shrinkage was caused by unsustainable water use in the region, but recent evidence suggests that air pollution from Europe which reduced rainfall in the Lake’s catchment area may also have contributed to this ecological disaster (Hwang et al., 2013). The Lake’s water level has risen since 2007, likely due, in part, to clean air regulations implemented by the European Union. Despite this positive turn around, air pollution continues to be a serious problem (Amegah and Agyei-Mensah, 2017) that threatens humans and wildlife throughout Africa.

An important form of air pollution is hydrocarbons, which are released during fossil fuel burning, particularly during transport, power generation, and other industrial activities (Karagulian et al., 2015). Pollution from airborne hydrocarbon compounds can sometimes be sensed without scientific equipment, by the bad smells, high air turbidity, and eye and lung irritation a person may experience in large cities with highly polluted air. When exposed to sunlight, these chemicals can react with other gases and particles in the atmosphere to produce photochemical smog, which is made up of ozone and other secondary compounds. In the upper atmosphere, ozone filters harmful ultraviolet radiation, which benefits most living things; but at ground level, high concentrations of ozone pose several dangers. For example, it damages plant tissues which make them brittle; high surface ozone levels have found to cause crop damage in Botswana and South Africa (Zunckel et al., 2004). Hydrocarbon exposure also poses several threats to humans: it altered some people’s DNA—often a cancer precursor—in Benin (Fanou et al., 2006), caused lung damage in Côte d’Ivoire (Kouassi et al., 2010), and subjected people to carcinogenic compounds in the DRC and Ghana (Tuakuila, 2013; Bortey-Sam et al., 2017). The lack of air monitoring and standards over much of Sub-Saharan (Petkova et al., 2013), and lack of awareness—people often confuse photochemical smog with natural mist and early-morning fog—should thus be of serious concern both to conservation biologists and society at large.
Air pollution from hydrocarbons often manifests itself as photochemical smog. Hanging like a thick cloud over industrial areas, people sometimes confuse it with natural mist and early-morning fog.
Burning fossil fuels also releases sulphur oxides (SOx) and nitrogen oxides (NOx) into the atmosphere, where they combine with water vapor to produce nitric and sulphuric acids. These acids later return to the ground as acid rain, with dramatically low pH relative to normal rainwater. Prevailing winds can transport acid rain clouds over long distances, so the effects of acid rain may occur hundreds of kilometres from its sources. Because the acid rain is closely tied to the water cycle, aquatic and soil organisms are particularly vulnerable to the negative effects of acid rain. Plants exposed to acid rain, either directly or after absorbing contaminated water from the ground, are often left severely weakened or even killed: it has even caused plant extirpations in Zambia (UNEP, 2006).
Another important contributor to air pollution is domestic fuel burning (Karagulian et al., 2015). During these activities, very small pollutant particles are released into the air. Because these particles are so small, they are difficult to filter from the air, and can easily be inhaled. Once inhaled, the particles can pass into the victim’s bloodstream, from where they negatively impact cardiovascular health, neurodevelopment, and cognitive function (WHO, 2013). Despite the harmful impact of these particles in the environment, their monitoring is virtually non-existent in Africa, making it very hard to guide air quality policy decisions and legislations. In contrast, measures that mitigate pollution from domestic fuel burning may even help slow the rate of habitat loss (Chapter 5), as this type of pollution is associated with inefficient wood stoves, slash-and-burn agriculture, and the artisanal charcoal industry.
Soil pollution
Soil pollution occurs when soil meets foreign chemicals and other pollutants. This type of pollution is often associated with industrial activities that extract resources from the earth, agricultural runoff, pesticide use, oil spills, acid rain, improper treatment of sewage, and improper disposal of waste. People and wildlife can then become sick through direct contact with contaminated soils, or through secondary contamination via polluted groundwater or eating food grown in contaminated soil. For example, a recent review reported how soil pollution has left medicinal plants toxic to humans in countries such as Botswana; Ghana; and Mali, at times with fatal consequences (Street, 2012).
The improper disposal of electronic waste (or e-waste in short) is a particularly serious form of soil pollution. Because electronic products contain toxic heavy metal contaminants that are expensive to recycle, discarded electronic products usually end up in dump yards (Figure 7.5). Here, open burning of electronic and other waste materials releases the toxic compounds into the soil, as well as the air and water (Robinson, 2009), from where it also accumulates in the environment.

Light pollution
Light pollution describes the addition of excessive, ill-timed, or poorly designed artificial light to the natural world. A consequence of an increasingly industrialized world (Falchi et al., 2016), light pollution has increased dramatically over the past decades as more people have gained greater access to electricity (Figure 7.6). Behavioural disruption is perhaps the most well-known consequence of increased light pollution—consider all the moths and other nocturnal insects (and insect predators, such as bats and geckos) attracted to artificial night lights. Light pollution also interferes with the navigation abilities of nocturnal species, which often use the stars, moon, and light reflectance from water surfaces to orientate themselves. For example, work in Gabon has shown how artificial lights disorientate sea turtle hatchlings trying to reach the sea (Bourgeois et al., 2009), while others have highlighted the significance of light-induced seabird mortality (Black, 2005). These and other behavioral disruptions—which include attraction to and repelling away from artificial light—may seem to only affect a small number of individuals around a few lights in your home. But the systemic impact of thousands of lights every night has wide-ranging ecosystem impacts when considering the cumulative impact of reduced reproductive performance (Firebaugh and Haynes, 2016), disrupted predator-prey dynamics (Minnaar et al., 2015) and disturbed night-time pollination services (Knop et al., 2017) on the many thousands of organisms impacted every night.

Light pollution also disrupts the natural day-night cycles with which most species evolved. These disruptions interfere with circadian rhythms, which negatively affect living organisms’ physiology. For example, one study showed that night-time light pollution disrupted natural sleep patterns in birds, leaving the affected individuals more susceptible to malaria infections (Ouyang et al., 2017). Circadian rhythm disruptions from light pollution (especially from high frequency “blue” light) also impact humans by increasing stress, fatigue, and anxiety, and susceptibility to obesity (Rybnikova et al., 2016) and cancer (Haim and Portnov, 2013). It is important to note that light pollution does not mean that the use of light is inherently bad—light has and will continue to play an important role in our daily lives. However, it does mean that we need to be more thoughtful about the consequences of light pollution and put measures in place to mitigate its impacts on the natural world and our own lives.
Noise pollution
Many people find a sense of freedom when they are in natural surroundings, with peace and quiet facilitating a much-needed connection to nature. These experiences are increasingly being threatened by noise pollution. However, noise pollution (also called acoustic pollution)—caused by human activities, such as industrial, military, and transportation systems—affects more than just the appealing tranquillity of nature. It also prevents animals from hearing each other, predators, and prey, all which could interfere with feeding, reproduction, navigation, and predator-avoidance behaviors. While African studies on the impact of noise pollution on wildlife are near-absent (Shannon et al., 2015), one study that did investigate the topic found that traffic noise increased dwarf mongooses’ (Helogale parvula, LC) alertness but also reduced responsiveness to alarm calls (Kern and Radford, 2016). Such responses could leave the affected individuals less fit and more vulnerable to predators.
Noise pollution prevents interferes with communication, feeding, reproduction, navigation, and predator-avoidance behaviors; it may even contribute to mass strandings of whales.
One would think that marine organisms living in the vast oceans may be spared from noise pollution, but this is not the case (Koper and Plön, 2012; Kunc et al., 2017). Sound carries much further in salt water than air, so noises from ship propellers; military sonar; seismic activities, and construction have significantly increased the level of ambient noise levels marine organisms experience. This increased level of ambient noise not only disrupts communication in sea animals (e.g. Cerchio et al., 2014), but can even lead to death (some mass whale strandings have been attributed to noise pollution: Morell et al., 2017; Williams et al., 2017). As with light pollution, there is a general need to be more thoughtful about the consequences of sound pollution on the natural world and to put measures in place (see e.g. Koper and Plön, 2012) to mitigate its impacts.
Thermal pollution
Thermal pollution describes localised human-induced temperature changes to the natural world. Aquatic ecosystems represent one of the ecosystems most vulnerable to thermal pollution. For example, when water is released from big dams, it comes from the colder middle and lower strata of the reservoir, leading to rapid cooling of aquatic ecosystems further downstream. The opposite is true at power plants that use river water as a coolant; turbines release their heat to the circulating water and then the warmed water is released back into the environment. These abrupt releases of thermally discordant water often lead to thermal shock which can be lethal to fish and other aquatic organisms. For example, studies from South Africa have shown that thermal shock can kill fish embryos and larvae and caused deformities in the young of Clanwilliam yellowfish (Barbus capensis, VU) (King et al., 1998).
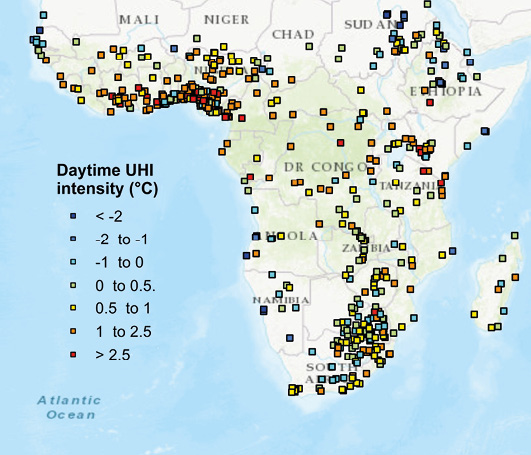
The urban heat island effect represents a terrestrial form of thermal pollution. Urban and other developed areas are generally covered with large swaths of man-made surfaces (e.g. asphalt roads, pavement surfaces, and building roofs), which absorb solar energy rather than reflect it. This absorbed heat, in combination with heat outputs from industrial activities, cause urban areas to function like “islands of heat” that are several degrees warmer (Figure 7.7) than surrounding rural areas (Feyisa et al., 2014; Chakraborty and Lee, 2018). The urban heat island effect reduces the quality of life for people and wildlife by reducing comfort and water availability (due to increasing evaporation). It also increases energy consumption to offset the heat increases which, in turn, contributes to air pollution and climate change.